The role of ATP-binding Cassette subfamily B member 6 in the inner ear
- PMID: 39557842
- PMCID: PMC11574312
- DOI: 10.1038/s41467-024-53663-x
The role of ATP-binding Cassette subfamily B member 6 in the inner ear
Abstract
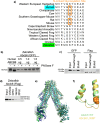
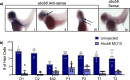
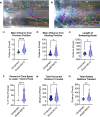
ABCB6 has been implicated in dyschromatosis universalis hereditaria, a condition characterized by hyperpigmented and hypopigmented skin macules. Dyschromatosis universalis hereditaria can also present with hearing loss. Dyschromatosis universalis hereditaria-associated mutations in ABCB6 have been reported, but the role of this protein in the inner ear has not been studied. Here we determine a high-resolution (2.93 Å) cryo-EM structure of ABCB6 and functionally characterized several dyschromatosis universalis hereditaria mutants. We find that the L356P mutant abolishes ABCB6 function, and affirm the underlying loss of ATP binding mechanism using molecular dynamics simulations based on our cryo-EM structure. To test the role of ABCB6 in the inner ear, we characterize Abcb6 (the ABCB6 homolog) in zebrafish. We show that Abcb6 suppression by morpholinos reduces inner ear and lateral line hair cell numbers. Morphants also lack the utricular otolith, which is associated with vestibular function. Co-injecting morpholinos with human ABCB6 mRNA partially rescues the morphant phenotype, suggesting that Abcb6 plays a developmental role in inner ear structures. Further, we show that Abcb6 knockout mice exhibit an increased auditory brainstem response threshold, resulting in reduced hearing sensitivity. Taken together, these data suggest ABCB6 plays a role in inner ear development and function.
© 2024. The Author(s).
Conflict of interest statement
Figures



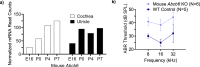
References
-
- Andolfo, I. et al. Missense mutations in the ABCB6 transporter cause dominant familial pseudohyperkalemia. Am. J. Hematol.88, 66–72 (2013). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 DC020701/DC/NIDCD NIH HHS/United States
- R01 CA194057, CA194206, P30 CA21745, CA21865, 5R01DK080834, NCI P30 CA021765, CA96832,/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R15 DC013900/DC/NIDCD NIH HHS/United States
- R01 NS116043/NS/NINDS NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

