G12/13-mediated signaling stimulates hepatic glucose production and has a major impact on whole body glucose homeostasis
- PMID: 39557854
- PMCID: PMC11574106
- DOI: 10.1038/s41467-024-54299-7
G12/13-mediated signaling stimulates hepatic glucose production and has a major impact on whole body glucose homeostasis
Abstract
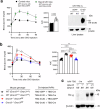
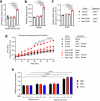
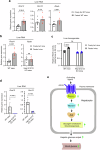
Altered hepatic glucose fluxes are critical during the pathogenesis of type 2 diabetes. G protein-coupled receptors represent important regulators of hepatic glucose production. Recent studies have shown that hepatocytes express GPCRs that can couple to G12/13, a subfamily of heterotrimeric G proteins that has attracted relatively little attention in the past. Here we show, by analyzing several mutant mouse strains, that selective activation of hepatocyte G12/13 signaling leads to pronounced hyperglycemia and that this effect involves the stimulation of the ROCK1-JNK signaling cascade. Using both mouse and human hepatocytes, we also show that activation of endogenous sphingosine-1-phosphate type 1 receptors strongly promotes glucose release in a G12/13-dependent fashion. Studies with human liver samples indicate that hepatic GNA12 (encoding Gα12) expression levels positively correlate with indices of insulin resistance and impaired glucose homeostasis, consistent with a potential pathophysiological role of enhanced hepatic G12/13 signaling.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK112698/DK/NIDDK NIH HHS/United States
- R01 DK129946/DK/NIDDK NIH HHS/United States
- R21 DK075021/DK/NIDDK NIH HHS/United States
- DK075021/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

