Comparative neurofilament light chain trajectories in CSF and plasma in autosomal dominant Alzheimer's disease
- PMID: 39557867
- PMCID: PMC11574007
- DOI: 10.1038/s41467-024-52937-8
Comparative neurofilament light chain trajectories in CSF and plasma in autosomal dominant Alzheimer's disease
Abstract
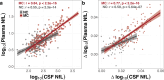
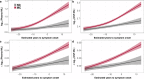
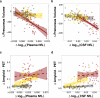
Disease-modifying therapies for Alzheimer's disease (AD) are likely to be most beneficial when initiated in the presymptomatic phase. To track the benefit of such interventions, fluid biomarkers are of great importance, with neurofilament light chain protein (NfL) showing promise for monitoring neurodegeneration and predicting cognitive outcomes. Here, we update and complement previous findings from the Dominantly Inherited Alzheimer Network Observational Study by using matched cross-sectional and longitudinal cerebrospinal fluid (CSF) and plasma samples from 567 individuals, allowing timely comparative analyses of CSF and blood trajectories across the entire disease spectrum. CSF and plasma trajectories were similar at presymptomatic stages, discriminating mutation carriers from non-carrier controls 10-20 years before the estimated onset of clinical symptoms, depending on the statistical model used. However, after symptom onset the rate of change in CSF NfL continued to increase steadily, whereas the rate of change in plasma NfL leveled off. Both plasma and CSF NfL changes were associated with grey-matter atrophy, but not with Aβ-PET changes, supporting a temporal decoupling of Aβ deposition and neurodegeneration. These observations support NfL in both CSF and blood as an early marker of neurodegeneration but suggest that NfL measured in the CSF may be better suited for monitoring clinical trial outcomes in symptomatic AD patients.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- AARF-21-846786/ALZ/Alzheimer's Association/United States
- K01 AG084816/AG/NIA NIH HHS/United States
- R01 AG071865/AG/NIA NIH HHS/United States
- K01AG084816/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG062667/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

