Enzymatically catalyzed molecular aggregation
- PMID: 39557870
- PMCID: PMC11574095
- DOI: 10.1038/s41467-024-54291-1
Enzymatically catalyzed molecular aggregation
Abstract
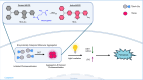
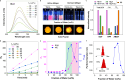
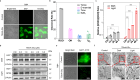
The dynamic modulation of the aggregation process of small molecules represents an important research objective for scientists. However, the complex and dynamic nature of internal environments in vivo impedes controllable aggregation processes of single molecules. In this study, we successfully achieve tumor-targeted aggregation of an aggregation-induced emission photosensitizer (AIE-PS), TBmA, with the catalysis of a tumor-overexpressed enzyme, γ-Glutamyl Transferase (GGT). Mechanistic investigations reveal that TBmA-Glu can be activated by GGT through cleavage of the γ-glutamyl bond and releasing TBmA. The poor water solubility of TBmA induces its aggregation, leading to aggregation-enhanced emission and photodynamic activities. The TBmA-Glu not only induces glutathione (GSH) depletion through GGT photo-degradation but also triggers lipid peroxidation accumulation and ferroptosis in cancer cells through photodynamic therapy. Finally, the in vivo studies conducted on female mice using both tumor xenograft and orthotopic liver cancer models have also demonstrated the significant anti-cancer effects of TBmA-Glu. The exceptional cancer-targeting ability and therapeutic efficiency demonstrated by this GGT activatable AIE-PS highlights enzymatic-mediated modulation as an effective approach for regulating small molecule aggregation intracellularly, thereby advancing innovative therapeutic strategies for various diseases.
© 2024. The Author(s).
Conflict of interest statement
Figures




References
-
- Van Regenmortel, M. H. V. Truth in science and in molecular recognition, post-truth in human affairs. J. Mol. Recognit.33, e2827 (2020). - PubMed
-
- Zhao, Z., Zhang, H., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission: new vistas at the aggregate level. Angew. Chem. Int. Ed.59, 9888–9907 (2020). - PubMed
-
- Yang, J., Fang, M. & Li, Z. Organic luminescent materials: the concentration on aggregates from aggregation‐induced emission. Aggregate1, 6–18 (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

