A modular platform for bioluminescent RNA tracking
- PMID: 39557883
- PMCID: PMC11574019
- DOI: 10.1038/s41467-024-54263-5
A modular platform for bioluminescent RNA tracking
Abstract
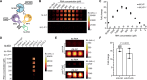
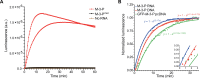
A complete understanding of RNA biology requires methods for tracking transcripts in vivo. Common strategies rely on fluorogenic probes that are limited in sensitivity, dynamic range, and depth of interrogation, owing to their need for excitation light and tissue autofluorescence. To overcome these challenges, we report a bioluminescent platform for serial imaging of RNAs. The RNA tags are engineered to recruit light-emitting luciferase fragments (termed RNA lanterns) upon transcription. Robust photon production is observed for RNA targets both in cells and in live animals. Importantly, only a single copy of the tag is necessary for sensitive detection, in sharp contrast to fluorescent platforms requiring multiple repeats. Overall, this work provides a foundational platform for visualizing RNA dynamics from the micro to the macro scale.
© 2024. The Author(s).
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

