Probing novel epitopes on the Plasmodium falciparum circumsporozoite protein for vaccine development
- PMID: 39557901
- PMCID: PMC11574195
- DOI: 10.1038/s41541-024-01006-8
Probing novel epitopes on the Plasmodium falciparum circumsporozoite protein for vaccine development
Abstract
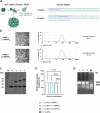
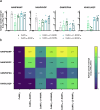
RTS,S and R21 are the only vaccines recommended by the WHO to protect children from Plasmodium falciparum (Pf) clinical malaria. Both vaccines target the Pf sporozoite surface protein circumsporozoite protein (CSP). Recent studies showed that human antibodies neutralize Pf sporozoites most efficiently when simultaneously binding to the PfCSP NANP repeat and the NPDP junction domain. However, neither RTS,S nor R21 targets this junction domain. To test the potential of the NPDP junction domain and other sites of PfCSP as innovative vaccine targets, we developed multiple vaccine candidates based on cucumber mosaic virus-like particles (CuMVTT-VLPs). These candidates vary in several aspects: the number of targeted NANP repeats, the presence or absence of the junction domain, the cleavage site, and up to three NVDP repeats within the target sequence. Immunogenicity and efficacy studies were conducted in BALB/c mice, utilizing chimeric Plasmodium berghei (Pb) sporozoites, in which the endogenous CSP has been replaced by PfCSP (Pb/PfCSP). We observed a positive association between the number of targeted NANP repeats and the induction of specific IgM/IgG antibodies. Elevated humoral responses led to enhanced protection against parasitemia after Pb/PfCSP sporozoite challenge. Especially high-avidity/affinity antibody formation and vaccine protection were NANP repeat-dependent. Intriguingly, vaccine efficacy was not enhanced by targeting sites on PfCSP other than the NANP repeats. Our data emphasize the dominant role of the NANP repeat region for induction of protective antibodies. Furthermore, we present here novel malaria vaccine candidates with an excellent immunogenic profile that confer sterile protection in mice, even in absence of adjuvants.
© 2024. The Author(s).
Conflict of interest statement
Figures




References
-
- World Health Organization (WHO). World Malaria Report 2023 (2023).
-
- Phillips, M. A. et al. Malaria. Nat. Rev. Dis. Prim. 3, 17050 (2017). - PubMed
-
- World Health Organization (WHO). World Malaria Report 2022 (2022).
Grants and funding
- 31003A_185114/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 31003A_185114/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 31003A_185114/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 31003A_185114/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 31003A_185114/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 31003A_185114/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- ID Grant 41-198/Universität Bern (University of Bern)
- ID Grant 41-198/Universität Bern (University of Bern)
- ID Grant 41-198/Universität Bern (University of Bern)
- ID Grant 41-198/Universität Bern (University of Bern)
- ID Grant 41-198/Universität Bern (University of Bern)
- ID Grant 41-198/Universität Bern (University of Bern)
- ID Grant 41-198/Universität Bern (University of Bern)
- ID Grant 41-198/Universität Bern (University of Bern)
LinkOut - more resources
Full Text Sources

