Exploring human pancreatic organoid modelling through single-cell RNA sequencing analysis
- PMID: 39558019
- PMCID: PMC11574267
- DOI: 10.1038/s42003-024-07193-3
Exploring human pancreatic organoid modelling through single-cell RNA sequencing analysis
Abstract
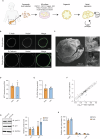
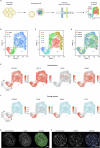
Human organoids have been proposed to be powerful tools mimicking the physiopathological processes of the organs of origin. Recently, human pancreatic organoids (hPOs) have gained increasing attention due to potential theragnostic and regenerative medicine applications. However, the cellular components of hPOs have not been defined precisely. In this work, we finely characterized these structures, focusing first on morphology and identity-defining molecular features under long-term culture conditions. Next, we focused our attention on hPOs cell type composition using single-cell RNA sequencing founding a complex heterogeneity in ductal components, ranging from progenitor components to terminally differentiated ducts. Furthermore, an extensive comparison of human pancreatic organoids with previously reported transcriptomics signature of human and mouse pancreatic ductal populations, confirmed the functional pancreatic duct subpopulation heterogeneity. Finally, we showed that pancreatic organoid cells follow a precise developmental trajectory and utilize diverse signalling mechanisms, including EGF and SPP1, to facilitate cell-cell communication and maturation. Together our results offer an in-depth description of human pancreatic organoids providing a strong foundation for future in vitro diagnostic and translational studies of pancreatic health and disease.
© 2024. The Author(s).
Conflict of interest statement
Figures





References
-
- Bouwens, L. & Pipeleers, D. G. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia41, 629–633 (1998). - PubMed
-
- Chong, S. K., Lindridge, J., Moniz, C. & Mowat, A. P. Exocrine pancreatic insufficiency in syndromic paucity of interlobular bile ducts. J. Pediatr. Gastroenterol. Nutr.9, 445–449 (1989). - PubMed
-
- Boxhoorn, L. et al. Acute pancreatitis. Lancet Lond. Engl.396, 726–734 (2020). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

