Coffee consumption is associated with intestinal Lawsonibacter asaccharolyticus abundance and prevalence across multiple cohorts
- PMID: 39558133
- PMCID: PMC11602726
- DOI: 10.1038/s41564-024-01858-9
Coffee consumption is associated with intestinal Lawsonibacter asaccharolyticus abundance and prevalence across multiple cohorts
Abstract
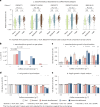
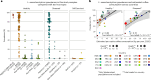
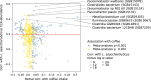
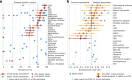
Although diet is a substantial determinant of the human gut microbiome, the interplay between specific foods and microbial community structure remains poorly understood. Coffee is a habitually consumed beverage with established metabolic and health benefits. We previously found that coffee is, among >150 items, the food showing the highest correlation with microbiome components. Here we conducted a multi-cohort, multi-omic analysis of US and UK populations with detailed dietary information from a total of 22,867 participants, which we then integrated with public data from 211 cohorts (N = 54,198). The link between coffee consumption and microbiome was highly reproducible across different populations (area under the curve of 0.89), largely driven by the presence and abundance of the species Lawsonibacter asaccharolyticus. Using in vitro experiments, we show that coffee can stimulate growth of L. asaccharolyticus. Plasma metabolomics on 438 samples identified several metabolites enriched among coffee consumers, with quinic acid and its potential derivatives associated with coffee and L. asaccharolyticus. This study reveals a metabolic link between a specific gut microorganism and a specific food item, providing a framework for the understanding of microbial dietary responses at the biochemical level.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: G.H., J.W. and T.D.S. are co-founders of ZOE. G.H., J.W., R.D., F.G. and K.M.B. are or have been employees of ZOE. N.S., F.A., S.E.B., C.H. and T.D.S. are consultants to ZOE. T.D.S., R.D., J.W., G.H., F.A., N.S. and S.E.B. receive options with ZOE. All other authors declare no competing interests.
Figures









References
-
- Liu, D. et al. Association of sugar-sweetened, artificially sweetened, and unsweetened coffee consumption with all-cause and cause-specific mortality: a large prospective cohort study. Ann. Intern. Med.175, 909–917 (2022). - PubMed
-
- O’Keefe, J. H. et al. Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all-cause mortality. J. Am. Coll. Cardiol.62, 1043–1051 (2013). - PubMed
-
- Muley, A., Muley, P. & Shah, M. Coffee to reduce risk of type 2 diabetes?: a systematic review. Curr. Diabetes Rev.8, 162–168 (2012). - PubMed
-
- van Dam, R. M. & Hu, F. B. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA294, 97–104 (2005). - PubMed
-
- Barré, T. et al. Elevated coffee consumption is associated with a lower risk of elevated liver fibrosis biomarkers in patients treated for chronic hepatitis B (ANRS CO22 Hepather cohort). Clin. Nutr.41, 610–619 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

