Selective recognition of RNA G-quadruplex in vitro and in cells by L-aptamer-D-oligonucleotide conjugate
- PMID: 39558155
- PMCID: PMC11662670
- DOI: 10.1093/nar/gkae1034
Selective recognition of RNA G-quadruplex in vitro and in cells by L-aptamer-D-oligonucleotide conjugate
Abstract
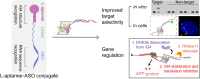
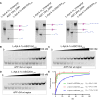
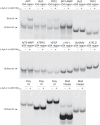
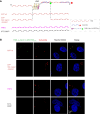
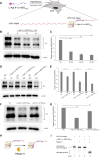
RNA Guanine-quadruplexes (rG4s) are important nucleic acid structures that govern vital biological processes. Although numerous tools have been developed to target rG4s, few specific tools are capable of discerning individual rG4 of interest. Herein, we design and synthesize the first L-aptamer-antisense oligonucleotide (ASO) conjugate, L-Apt.4-1c-ASO15nt(APP), with a focus on recognizing the amyloid precursor protein (APP) rG4 region as an example. The L-aptamer module binds with the rG4 structure, whereas ASO hybridizes with flanking sequences. Together, these two modules enhance the precise recognition of APP rG4. We demonstrate that the L-Apt.4-1c-ASO15nt(APP) conjugate can interact with the APP rG4 region with sub-nanomolar binding affinity, and distinguish APP rG4 from other G4s and non-G4s in vitro and in cells. We also show that L-Apt.4-1c-ASO15nt(APP) can inhibit APP protein expression. Notably, we investigate the inhibitory mechanism of this newly developed tool, and reveal that it controls gene expression by hindering DHX36 protein from unraveling the rG4, as well as by promoting translational inhibition and RNase H-mediated mRNA knockdown activity. Our novel L-aptamer-ASO conjugate tool not only enables the specific recognition of rG4 region of interest, but also allows efficient gene control via targeting rG4-containing transcripts in cells.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

