Artificial intelligence-based personalized survival prediction using clinical and radiomics features in patients with advanced non-small cell lung cancer
- PMID: 39558311
- PMCID: PMC11572056
- DOI: 10.1186/s12885-024-13190-w
Artificial intelligence-based personalized survival prediction using clinical and radiomics features in patients with advanced non-small cell lung cancer
Abstract
Background: Multiple first-line treatment options have been developed for advanced non-small cell lung cancer (NSCLC) in each subgroup determined by predictive biomarkers, specifically driver oncogene and programmed cell death ligand-1 (PD-L1) status. However, the methodology for optimal treatment selection in individual patients is not established. This study aimed to develop artificial intelligence (AI)-based personalized survival prediction model according to treatment selection.
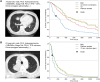
Methods: The prediction model was built based on random survival forest (RSF) algorithm using patient characteristics, anticancer treatment histories, and radiomics features of the primary tumor. The predictive accuracy was validated with external test data and compared with that of cox proportional hazard (CPH) model.
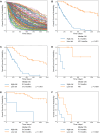
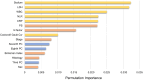
Results: A total of 459 patients (training, n = 299; test, n = 160) with advanced NSCLC were enrolled. The algorithm identified following features as significant factors associated with survival: age, sex, performance status, Brinkman index, comorbidity of chronic obstructive pulmonary disease, histology, stage, driver oncogene status, tumor PD-L1 expression, administered anticancer agent, six markers of blood test (sodium, lactate dehydrogenase, etc.), and three radiomics features associated with tumor texture, volume, and shape. The C-index of RSF model for test data was 0.841, which was higher than that of CPH model (0.775, P < 0.001). Furthermore, the RSF model enabled to identify poor survivor treated with pembrolizumab because of tumor PD-L1 high expression and those treated with driver oncogene targeted therapy according to driver oncogene status.
Conclusions: The proposed AI-based algorithm accurately predicted the survival of each patient with advanced NSCLC. The AI-based methodology will contribute to personalized medicine.
Trial registration: The trial design was retrospectively registered study performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Nagoya University Graduate School of Medicine (approval: 2020 - 0287).
Keywords: Artificial intelligence; Machine learning; Non-small cell lung cancer; Precision medicine; Random survival forest.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

