miR-23b-3p, miR-126-3p and GAS5 delivered by extracellular vesicles inhibit breast cancer xenografts in zebrafish
- PMID: 39558342
- PMCID: PMC11572517
- DOI: 10.1186/s12964-024-01936-9
miR-23b-3p, miR-126-3p and GAS5 delivered by extracellular vesicles inhibit breast cancer xenografts in zebrafish
Abstract
Background: Extracellular vesicles (EVs) are a group of nanoscale cell-derived membranous structures secreted by all cell types, containing molecular cargoes involved in intercellular communication. EVs can be used to mimic "nature's delivery system" to transport nucleic acids, peptides, lipids, and metabolites to target recipient cells. EVs offer a range of advantages over traditional synthetic carriers, thus paving the way for innovative drug delivery approaches that can be used in different diseases, including cancer. Here, by using breast cancer (BC) cells treated with the multi-kinase inhibitor sorafenib, we generated EVs enriched in specific non-coding RNAs (miR-23b-3p, miR-126-3p, and the long ncRNA GAS5) and investigated their potential impact on the aggressive properties of the BC in vitro and in vivo using zebrafish.
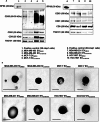
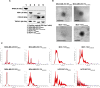
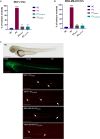
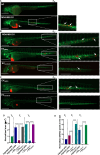
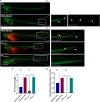
Methods: EVs were collected from 4 different BC cell lines (HCC1937, MDA-MB-231, MCF-7, and MDA-MB-453) and characterized by western blotting, transmission electron microscopy and nanoparticle tracking analysis. Levels of encapsulated miR-23b-3p, miR-126-3p, and GAS5 were quantified by ddPCR. The role of the EVs as carriers of ncRNAs in vivo was established by injecting MDA-MB-231 and MDA-MB-453 cells into zebrafish embryos followed by EV-based treatment of the xenografts with EVs rich in miR-23b-3p, miR-126-3p and GAS5.
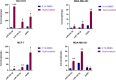
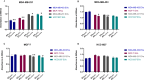
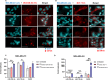
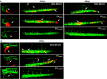
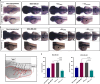
Results: ddPCR analysis revealed elevated levels of miR-23b-3p, miR-126-3p, and GAS5, encapsulated in the EVs released by the aforementioned cell lines, following sorafenib treatment. The use of EVs as carriers of these specific ncRNAs in the treatment of BC cells resulted in a significant increase in the expression levels of the three ncRNAs along with the inhibition of cellular proliferation in vitro. In vivo experiments demonstrated a remarkable reduction of xenograft tumor area, suppression of angiogenesis, and decreased number of micrometastasis in the tails after administration of EVs enriched with these ncRNAs.
Conclusions: Our study demonstrated that sorafenib-induced EVs, enriched with specific tumor-suppressor ncRNAs, can effectively inhibit the aggressive BC characteristics in vitro and in vivo. Our findings indicate an alternative way to enrich EVs with specific tumor-suppressor ncRNAs by treating the cells with an anticancer drug and support the development of new potential experimental molecular approaches to target the aggressive properties of cancer cells.
Keywords: Angiogenesis; Breast cancer; EVs; Zebrafish xenograft; ddPCR; ncRNAs.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- Bray Bsc F et al. Laversanne | Mathieu, Hyuna |, Phd S, Ferlay J, Siegel Mph RL,. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2024 [cited 2024 Jun 3]; https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21834 - PubMed
-
- Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1). - PubMed
-
- Waks AG, Winer EP. Breast Cancer Treatment: a review. JAMA. 2019;321(3):288–300. - PubMed
-
- Lee Y, Graham P, Li Y. Extracellular vesicles as a novel approach for breast cancer therapeutics. Cancer Letters. Volume 555. Elsevier Ireland Ltd; 2023. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

