Cooperative participation of CagA and NFATc1 in the pathogenesis of antibiotics-responsive gastric MALT lymphoma
- PMID: 39558403
- PMCID: PMC11575159
- DOI: 10.1186/s12935-024-03552-6
Cooperative participation of CagA and NFATc1 in the pathogenesis of antibiotics-responsive gastric MALT lymphoma
Abstract
Background: This study aimed to explore whether cytotoxin-associated gene A (CagA) can inhibit cell cycle progression by activating nuclear factor of activated T cells (NFAT) in lymphoma B cells and contribute to Helicobacter pylori eradication (HPE) responsiveness (complete remission [CR] after HPE) in gastric mucosa-associated lymphoid tissue (MALT) lymphoma.
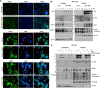
Materials and methods: We co-cultured three B-lymphoma cell lines (MA-1, OCI-Ly3, and OCI-Ly7) with HP strains (derived from HPE-responsive gastric MALT lymphoma) and evaluated the expression patterns of CagA, phosphorylated (p)-CagA (CagAP-Tyr), and CagA-signaling molecules, cell-cycle inhibitors, p-NFATc1 (Ser172), and NFATc1 using western blotting. Furthermore, we evaluated the association between nuclear NFATc1 expression in the tumor cells of 91 patients who received first-line HPE (59 patients with HPE responsiveness and 32 without HPE responsiveness) and HPE responsiveness and CagA expression in tumor cells.
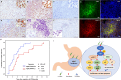
Results: In HP strains co-cultured with B cell lymphoma cell lines, CagA was translocated to the nucleus through tyrosine phosphorylation (CagAP-Tyr) and simultaneously dephosphorylated NFATc1, subsequently causing nuclear NFATc1 translocation and stimulating the expression of p-SHP-2/p-ERK/Bcl-xL. Activated NFATc1 causes G1 cell cycle retardation in both MA-1 and OCI-Ly3 cells by triggering p21 and p27 production. Nuclear NFATc1 localization was significantly associated with the presence of CagA in gastric MALT lymphomas (80% [41/51] vs. 33% [13/40]; p < 0.001) and with HPE responsiveness (73% [43/59] vs. 25% [8/32]; p < 0.001). Patients exhibiting both the presence of CagA and nuclear NFATc1 localization responded more rapidly to HPE than those without (median interval to CR, 4.00 vs. 6.00 months, p = 0.003).
Conclusions: Our findings indicated that CagA and NFATc1 cooperatively participate in the lymphomagenesis of HPE-responsive gastric MALT lymphoma.
Keywords: Helicobacter pylori; CagA; MALT lymphoma; NFATc1; Stomach.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
-
- Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol. 2002;3(2):97–104. - PubMed
-
- Zullo A, Hassan C, Cristofari F, et al. Effects of helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissuelymphoma. Clin Gastroenterol Hepatol. 2010;8(2):105–10. - PubMed
-
- Kuo SH, Cheng AL. Helicobacter pylori and mucosa-associated lymphoid tissue: what’s new. Hematology Am Soc Hematol Educ Program. 2013;2013:109–17. - PubMed
-
- Farinha P, Gascoyne RD. Helicobacter pylori and MALT lymphoma. Gastroenterology. 2005;128(6):1579–605. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

