IL-13 inhibition in the treatment of atopic dermatitis - new and emerging biologic agents
- PMID: 39558725
- PMCID: PMC11574908
- DOI: 10.1177/03000605241286832
IL-13 inhibition in the treatment of atopic dermatitis - new and emerging biologic agents
Abstract
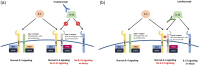
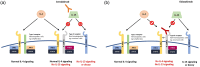
Atopic dermatitis (AD) is a common, chronic, and recurrent inflammatory skin condition that affects a considerable portion of the population, and is particularly prevalent among children. The development of AD is influenced by environmental and genetic factors, which cause epidermal barrier dysfunction, immune dysregulation, and dysbiosis. In immune dysregulation, there is excessive production of cytokines. Among the cytokines, interleukin (IL)-13 plays a major role in the pathogenesis of AD. Searching for new and more selective treatments for moderate-to-severe cases is important because of the considerable effect of AD on the quality of life. Tralokinumab and lebrikizumab are selective IL-13 inhibitors that have demonstrated safety and efficacy as treatment options for AD in phase III trials. Tralokinumab is approved for use in Europe and the USA, while lebrikizumab is approved only in Europe. Cendakimab, which is another IL-13 selective inhibitor, has shown promising results in phase II trials, providing safe and effective outcomes. Eblasakimab, which disrupts IL-13 and IL-4 signaling pathways, is currently in phase II trials following well-tolerated administration in phase I studies. This narrative review aims to outline the current state of knowledge regarding the effectiveness and safety of these four biologic agents targeting IL-13 signaling.
Keywords: Atopic dermatitis; cendakimab; eblasakimab; interleukin-13; lebrikizumab; monoclonal antibody; tralokinumab.
Conflict of interest statement
Declaration of conflicting interestOrhan Yilmaz, Carlos Teixeira, and Diana Bernardo declare that there is no conflict of interest. Tiago Torres has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials sponsored by AbbVie, Amgen, Almirall, Amgen, Arena Pharmaceuticals, Biocad, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Fresenius-Kabi, Janssen, LEO Pharma, Eli Lilly, MSD, Mylan, Novartis, Pfizer, Samsung-Bioepis, Sanofi-Genzyme, Sandoz, and UCB.
Figures
References
-
- Amat F, Soria A, Tallon P, et al.. New insights into the phenotypes of atopic dermatitis linked with allergies and asthma in children: An overview. Clin Exp Allergy 2018; 48: 919–934. DOI: 10.1111/cea.13156. - PubMed
-
- Chiricozzi A, Gori N, Maurelli M, et al.. Biological agents targeting interleukin-13 for atopic dermatitis. Expert Opin Biol Ther 2022; 22: 651–659. DOI: 10.1080/14712598.2022.2035356. - PubMed
-
- Weidinger S, Beck LA, Bieber T, et al.. Atopic dermatitis. Nat Rev Dis Primers 2018; 4: 1. DOI: 10.1038/s41572-018-0001-z. - PubMed
-
- Rønnstad ATM, Halling-Overgaard AS, Hamann CR, et al.. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: A systematic review and meta-analysis. J Am Acad Dermatol 2018; 79: 448–456.e30. DOI: 10.1016/j.jaad.2018.03.017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

