Plasma p-tau181 and GFAP reflect 7T MR-derived changes in Alzheimer's disease: A longitudinal study of structural and functional MRI and MRS
- PMID: 39558898
- PMCID: PMC11667506
- DOI: 10.1002/alz.14318
Plasma p-tau181 and GFAP reflect 7T MR-derived changes in Alzheimer's disease: A longitudinal study of structural and functional MRI and MRS
Abstract
Background: Associations between longitudinal changes of plasma biomarkers and cerebral magnetic resonance (MR)-derived measurements in Alzheimer's disease (AD) remain unclear.
Methods: In a study population (n = 127) of healthy older adults and patients within the AD continuum, we examined associations between longitudinal plasma amyloid beta 42/40 ratio, tau phosphorylated at threonine 181 (p-tau181), glial fibrillary acidic protein (GFAP), neurofilament light chain (NfL), and 7T structural and functional MR imaging and spectroscopy using linear mixed models.
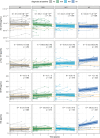
Results: Increases in both p-tau181 and GFAP showed the strongest associations to 7T MR-derived measurements, particularly with decreasing parietal cortical thickness, decreasing connectivity of the salience network, and increasing neuroinflammation as determined by MR spectroscopy (MRS) myo-inositol.
Discussion: Both plasma p-tau181 and GFAP appear to reflect disease progression, as indicated by 7T MR-derived brain changes which are not limited to areas known to be affected by tau pathology and neuroinflammation measured by MRS myo-inositol, respectively.
Highlights: This study leverages high-resolution 7T magnetic resonance (MR) imaging and MR spectroscopy (MRS) for Alzheimer's disease (AD) plasma biomarker insights. Tau phosphorylated at threonine 181 (p-tau181) and glial fibrillary acidic protein (GFAP) showed the largest changes over time, particularly in the AD group. p-tau181 and GFAP are robust in reflecting 7T MR-based changes in AD. The strongest associations were for frontal/parietal MR changes and MRS neuroinflammation.
Keywords: 7 Tesla; Alzheimer's disease; NeuroMET Memory Metric; amyloid beta 42/40; blood‐based biomarkers; functional magnetic resonance imaging; glial fibrillary acidic protein; magnetic resonance imaging; magnetic resonance spectroscopy; memory; mild cognitive impairment; neurofilament light chain; plasma biomarkers; subjective cognitive decline; tau phosphorylated at threonine 181.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Charlotte E. Teunissen has research contracts with Acumen, ADx Neurosciences, AC‐Immune, Alamar, Aribio, Axon Neurosciences, Beckman‐Coulter, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, Cognition Therapeutics, EIP Pharma, Eisai, Eli Lilly, Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, Olink, PeopleBio, Quanterix, Roche, Siemens, Toyama, and Vivoryon. Charlotte E. Teunissen is editor‐in‐chief of
Figures





References
-
- Lantero Rodriguez J, Karikari TK, Suarez‐Calvet M, et al. Plasma p‐tau181 accurately predicts Alzheimer's disease pathology at least 8 years prior to post‐mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140(3):267‐278. doi:10.1007/s00401-020-02195-x - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 15HLT04NeuroMET/EMPIR programme, co-financed by the Participating States and from the European Union's Horizon 2020 research and innovation programme
- 18HLT09NeuroMET2/EMPIR programme, co-financed by the Participating States and from the European Union's Horizon 2020 research and innovation programme
- 22HLT07NEuroBioSTand/EMPIR programme, co-financed by the Participating States and from the European Union's Horizon 2020 research and innovation programme
- 327654276-SFB1315/German Research Foundation
- Fondation pour la Recherche Médicale
- PPP-allowance;#LSHM20106/Topsector Life Sciences & Health
- Alzheimer Nederland
- Selfridges Group Foundation
- Alzheimer's Drug Discovery Foundation
- #73305095007/Dutch Research Council (ZonMW)
- National Multiple Sclerosis Society
- 101034344/EPND (IMI 2 Joint Undertaking)
- 831434/Innovative Medicines Initiative 3TR Horizon 2020
- 860197(MIRIADE)/Marie Curie international training network
- JPND (bPRIDE)
- Health Holland
- ALZ/Alzheimer's Association/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

