Rapid Desensitization to Antitumoral Agents. Result from a Retrospective Study, DESARCh
- PMID: 39558941
- PMCID: PMC11569765
- DOI: 10.1177/00185787241278702
Rapid Desensitization to Antitumoral Agents. Result from a Retrospective Study, DESARCh
Abstract
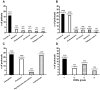
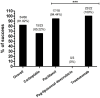
Antitumoral drugs (ADs) can induce drug hypersensitivity reactions (DHRs). Rapid drug desensitization (RDD) protocols represent an important option to mitigate recurrent DHRs thus allowing the safe administration of ADs at therapeutic doses. The aim of this retrospective study was to assess the effectiveness of the RDD protocols performed at our institution. The "DESARCh" study was a retrospective, observational study that included consecutive patients who underwent RDD protocols from January 2011 to December 2022 at IRCCS Ospedale Sacro Cuore Don Calabria in Negrar di Valpolicella, Verona, Italy. The RDD protocol consisted of a 5-step protocol with 5 different concentrations of the drugs at 1:1, 1:10, 1:100, 1:1,000 and 1:10,000 dilution given intravenously over a 1-hour infusion each, with concentrations increasing from the most diluted to the most concentrated form, preceded by a 30-min premedication regimen. A total of 66 RDD protocols were administered to 25 female patients with ovarian (64%; n = 16/25), breast (12%; n = 3/25), endometrium (8%; n = 2/25), cervix (8%; n = 2/25), uterine (4%; n = 1/25) and fallopian tubes (4%; n = 1/25) cancers. A known history of atopy/allergy was reported by 36% (n = 9/25) of patients. Patients received RDD protocols because of DHRs to carboplatin (n = 23/66, 34.85%), paclitaxel (n = 18/66, 27.27%), pegylated liposomal doxorubicin (n = 3/66, 4.55%), and trastuzumab (n = 22/66, 33.33%). DHRs were mild-moderate, severe and life-threatening in 60.72%, 28.57% and 10.71% of cases, respectively. The success rate of RDD protocols, defined as the rate of complete administration of full target dose with no breakthrough reactions, was 81.82% (n = 54/66). Success rate was lower for carboplatin compared to other drugs (65.22% vs 90.7%; P = .017678). The RDD protocol used in our institution was found to be safe, with a meaningful success rate. However, further research is needed to better understand the underlying mechanisms of DHRs and to enhance effectiveness, particularly for patients experiencing DHRs to platinum compounds. This study was approved by the ethics committee of Verona and Rovigo (Italy) with approval number 15476 on 10/03/2023 and it was registered with the Register of Observational Studies of the Italian Medicines Agency (AIFA) (available since 31 January 2023), with ID n. 109, on 28/02/2023 (https://www.aifa.gov.it/en/registro-studi-osservazionali).
Keywords: antitumoral drugs; desensitization procedures; hospital pharmacy; hypersensitivity reactions; “DESARCh” study.
© The Author(s) 2024.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

