Relative fitness of wild-type and phage-resistant pyomelanogenic P. aeruginosa and effects of combinatorial therapy on resistant formation
- PMID: 39559211
- PMCID: PMC11570307
- DOI: 10.1016/j.heliyon.2024.e40076
Relative fitness of wild-type and phage-resistant pyomelanogenic P. aeruginosa and effects of combinatorial therapy on resistant formation
Abstract
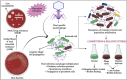
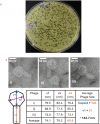
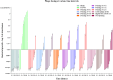
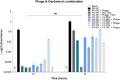
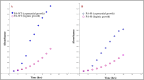
Bacteriophages, the natural predators of bacteria, are incredibly potent candidates to counteract antimicrobial resistance (AMR). However, the rapid development of phage-resistant mutants challenges the potential of phage therapy. Understanding the mechanisms of bacterial adaptations to phage predation is crucial for phage-based prognostic applications. Phage cocktails and combinatorial therapy, using optimized dosage patterns of antibiotics, can negate the development of phage-resistant mutations and prolong therapeutic efficacy. In this study, we describe the characterization of a novel bacteriophage and the physiology of phage-resistant mutant developed during infection. M12PA is a P. aeruginosa-infecting bacteriophage with Myoviridae morphology. We observed that prolonged exposure of P. aeruginosa to M12PA resulted in the selection of phage-resistant mutants. Among the resistant mutants, pyomelanin-producing mutants, named PA-M, were developed at a frequency of 1 in 16. Compared to the wild-type, we show that PA-M mutant is severely defective in virulence properties, with altered motility, biofilm formation, growth rate, and antibiotic resistance profile. The PA-M mutant exhibited reduced pathogenesis in an allantoic-infected chick embryo model system compared to the wild-type. Finally, we provide evidence that combinatory therapy, combining M12PA with antibiotics or other phages, significantly delayed the emergence of resistant mutants. In conclusion, our study highlights the potential of combinatory phage therapy to delay the development of phage-resistant mutants and enhance the efficacy of phage-based treatments against P. aeruginosa.
Keywords: Anti-phage mutation; Antibiotics; Antimicrobial resistance; Phage cocktails; Pigments; Pyomelanin.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Ghasemian A., Mobarez A.M., Peerayeh S.N., Abadi A.T.B., Khodaparast S., Nojoomi F. Report of plasmid-mediated colistin resistance in Klebsiella oxytoca from Iran. Reviews and Research in Medical Microbiology. Apr. 2018;29(2):59. doi: 10.1097/MRM.0000000000000134. - DOI
-
- Karn S.L., Gangwar M., Kumar R., Bhartiya S.K., Nath G. Phage therapy: a revolutionary shift in the management of bacterial infections, pioneering new horizons in clinical practice, and reimagining the arsenal against microbial pathogens. Front. Med. Oct. 2023;10 doi: 10.3389/fmed.2023.1209782. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

