Perplexing paradoxical reactions: navigating the complexity of protracted tuberculosis meningitis-a case report
- PMID: 39559357
- PMCID: PMC11570994
- DOI: 10.3389/fimmu.2024.1441945
Perplexing paradoxical reactions: navigating the complexity of protracted tuberculosis meningitis-a case report
Abstract
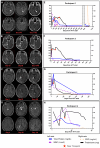
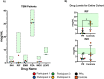
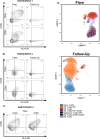
Tuberculous meningitis (TBM) has considerable mortality and morbidity, and it often presents therapeutic challenges when complicated by paradoxical reactions (PRs). Here, the clinical course of four cases of TBM patients complicated by PRs in a longitudinal TB cohort is described while also providing insights from the larger clinical cohort. Research flow cytometry, biomarker analysis, and drug concentrations were performed on available samples. All participants were initiated on standard antituberculosis therapy (ATT) and enrolled at the onset of PRs (PR group) or 2-4 months after the start of ATT (controls). The four TBM participants highlighted here presented with fevers, headaches, neurological deficits, and fatigue at the initial presentation. Upon diagnosis, all were initiated on rifampin, isoniazid, pyrazinamide, and ethambutol (RHZE) at standard doses and on corticosteroids. The median time to first PR was 37 days with recrudescence of initial TBM signs and symptoms at the time of PR. At the time of referral, all participants had low drug concentrations requiring dose optimization and regimen intensification as well as recrudescent flares upon corticosteroid taper, with one individual developing enlargement of tuberculoma 1 year following completion of ATT. Based on biomarkers and flow cytometry, PRs are characterized by elevated interferon-gamma and ferritin levels in the plasma compared to controls. In the TBM participants, T-cell activation with elevated levels of inflammatory biomarkers in the cerebrospinal fluid (CSF) was seen at the time of PR. These unique and highly detailed TBM cases provide insights into the pathogenesis of PRs, which may assist with future diagnostics and treatment.
Keywords: case series; immunocompetent; paradoxical reaction; therapeutic drug monitoring; tuberculous meningitis.
Copyright © 2024 Gooding, Hammoud, Epling, Rocco, Laidlaw, Kuriakose, Chaturvedi, Galindo, Ma, Mystakelis, Poole, Russo, Shah, Malone, Rupert, Sereti and Manion.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Floyd K, Adam T, Bastard M, Gebraselassie N, Yamanaka T. Global Tuberculosis Report. Geneva: World Health Organization; (2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

