Recent advances in transition-metal-free arylation reactions involving hypervalent iodine salts
- PMID: 39559439
- PMCID: PMC11572100
- DOI: 10.3762/bjoc.20.243
Recent advances in transition-metal-free arylation reactions involving hypervalent iodine salts
Abstract
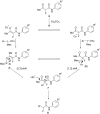
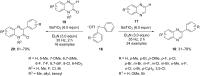
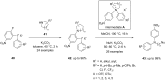
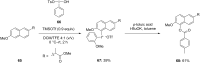
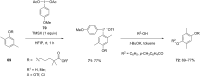
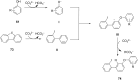
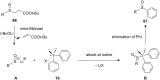
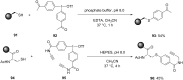
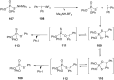
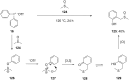
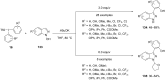
Diaryliodonium salts have become widely recognized as arylating agents in the last two decades. Both, symmetrical and unsymmetrical forms of these salts serve as effective electrophilic arylating reagents in various organic syntheses. The use of diaryliodoniums in C-C and carbon-heteroatom bond formations, particularly under metal-free conditions, has further enhanced the popularity of these reagents. In this review, we concentrate on various arylation reactions involving carbon and other heteroatoms, encompassing rearrangement reactions in the absence of any metal catalyst, and summarize advancements made in the last five years.
Keywords: arylation reaction; diaryliodonium salts; electrophilic arylation reagent; metal-free arylation; rearrangement reaction.
Copyright © 2024, Mamgain et al.
Figures


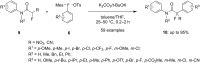


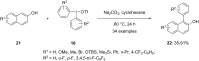









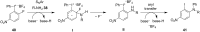



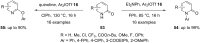








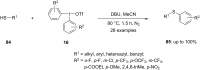
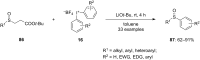



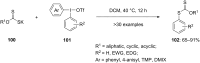
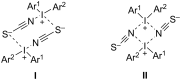
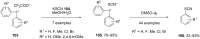
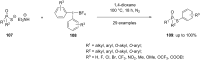
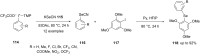



Similar articles
-
Recent Advances in Transition-Metal-Free Late-Stage C-H and N-H Arylation of Heteroarenes Using Diaryliodonium Salts.Pharmaceuticals (Basel). 2021 Jul 11;14(7):661. doi: 10.3390/ph14070661. Pharmaceuticals (Basel). 2021. PMID: 34358087 Free PMC article. Review.
-
Iodine(III) Reagents in Radical Chemistry.Acc Chem Res. 2017 Jul 18;50(7):1712-1724. doi: 10.1021/acs.accounts.7b00148. Epub 2017 Jun 21. Acc Chem Res. 2017. PMID: 28636313 Free PMC article.
-
Aryl Transfer Selectivity in Metal-Free Reactions of Unsymmetrical Diaryliodonium Salts.Chemistry. 2017 Nov 13;23(63):15852-15863. doi: 10.1002/chem.201702732. Epub 2017 Sep 1. Chemistry. 2017. PMID: 28793179 Review.
-
Visible Light Mediated Photoredox Catalytic Arylation Reactions.Acc Chem Res. 2016 Aug 16;49(8):1566-77. doi: 10.1021/acs.accounts.6b00229. Epub 2016 Aug 2. Acc Chem Res. 2016. PMID: 27482835
-
Arylation with unsymmetrical diaryliodonium salts: a chemoselectivity study.Chemistry. 2013 Jul 29;19(31):10334-42. doi: 10.1002/chem.201300860. Epub 2013 Jun 20. Chemistry. 2013. PMID: 23788251 Free PMC article.
Cited by
-
Advances in α-Arylation of Carbonyl Compounds: Diaryliodonium Salts as Arylating Agents.Molecules. 2025 Jul 18;30(14):3019. doi: 10.3390/molecules30143019. Molecules. 2025. PMID: 40733286 Free PMC article. Review.
-
Dibenzothiophenium Salts: Practical Alternatives to Hypervalent I(III)-Based Reagents.Acc Chem Res. 2025 Feb 18;58(4):635-646. doi: 10.1021/acs.accounts.4c00804. Epub 2025 Feb 2. Acc Chem Res. 2025. PMID: 39895033 Free PMC article.
-
Photoredox-catalyzed arylation of isonitriles by diaryliodonium salts towards benzamides.Beilstein J Org Chem. 2025 Jul 21;21:1480-1488. doi: 10.3762/bjoc.21.110. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 40726597 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
