Quadrivalent HPV (4vHPV) vaccine immunogenicity and safety in women using immunosuppressive drugs due to solid organ transplant
- PMID: 39559707
- PMCID: PMC11570996
- DOI: 10.3389/fcimb.2024.1452916
Quadrivalent HPV (4vHPV) vaccine immunogenicity and safety in women using immunosuppressive drugs due to solid organ transplant
Abstract
Introduction: Immunocompromised persons are at high risk of persistent Human Papilloma Virus (HPV) infection and associated diseases. Few studies evaluated HPV vaccines in immunocompromised persons. This study aimed to evaluate the quadrivalent HPV vaccine (4vHPV) immunogenicity and safety in solid organ transplant (SOT) recipients, in comparison to immunocompetent women (IC).
Methods: Open-label clinical trial that enrolled SOT recipients and immunocompetent women aged 18 to 45 years. All participants received three doses of 4vHPV vaccine. Blood samples were drawn for evaluation of immune responses at baseline and one month after the third vaccination. Seroconversion rates and antibody geometric mean concentration (GMC) against HPV 6, 11, 16, 18, 31, 35, 52 and 58 were measured with in-house multiplexed serology assay (xMAP technology). Follow-up for the local and systemic adverse events (AEs) continued for seven days after each vaccination. Severe AEs were evaluated throughout the study.
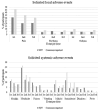
Results: 125 SOT and 132 immunocompetent women were enrolled; 105 (84%) SOT and 119 (90%) immunocompetent women completed the study. At baseline, HPV seropositivity was not significantly different between groups. Seroconversion rates were significantly lower in SOT (HPV18, 57%; HPV6 and 16, 69%; and HPV11, 72%) than in immunocompetent women (100% seroconversion to all vaccine types) (p<0.001). Antibody GMCs of all four HPV vaccine types were also significantly lower in SOT (p<0.001). Pain in the injection site and headache were the most frequent adverse event in both groups. Local pain was more frequent in immunocompetent women than in SOT recipients. Rates of other AEs were comparable in both groups.
Conclusion: 4vHPV vaccine was well-tolerated by SOT recipients. We found strong evidence of lower humoral immune responses to 4vHPV vaccine in SOT compared to immunocompetent women, which strengthen recommendation of routine cervical cancer screening in SOT recipients regardless of HPV vaccination status.
Keywords: cancer prevention; immunogenicity; immunosuppression; papillomavirus vaccines; safety; solid organ transplant.
Copyright © 2024 Miyaji, Infante, Picone, Dillner, Kann, Eklund, Levi, de Oliveira, Lara, Kawakami, Tacla, Castanheira, Mayaud and Sartori.
Conflict of interest statement
Since June 2020, VI is an employee of Instituto Butantan and since March 2021, KM is an employee of Instituto Butantan producer of 4vHPV vaccine in Brazil, through a technology transfer agreement. CR is an employee of Instituto Butantan since April 2023. AL, AS and CP received grant from Instituto Butantan in 2021-2022, to conduct studies on COVID vaccines. Data collection for this study and analysis were performed before that. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Boey L., Curinckx A., Roelants M., Derdelinckx I., Van Wijngaerden E., De Munter P., et al. . (2021). Immunogenicity and safety of the 9-Valent human papillomavirus vaccine in solid organ transplant recipients and adults infected with human immunodeficiency virus (HIV). Clin. Infect. Dis. 73, e661–e671. doi: 10.1093/cid/ciaa1897 - DOI - PubMed
-
- Bogani G., Sopracordevole F., Ciavattini A., Ghelardi A., Vizza E., Vercellini P., et al. . (2024). HPV-related lesions after hysterectomy for high-grade cervical intraepithelial neoplasia and early-stage cervical cancer: A focus on the potential role of vaccination. Tumori 110, 139–145. doi: 10.1177/03008916231208344 - DOI - PubMed
-
- D’Augè T. G., Cuccu I., Etrusco A., D'Amato A., Laganà A. S., D'Oria O., et al. . (2024). State of the art on HPV-related cervical lesions. Ital. J. Gynæcology Obstetrics 36, 135–137. doi: 10.36129/jog.2023.144 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

