A novel mitochondrial pyruvate carrier inhibitor drives stem cell-like memory CAR T cell generation and enhances antitumor efficacy
- PMID: 39559715
- PMCID: PMC11570499
- DOI: 10.1016/j.omton.2024.200897
A novel mitochondrial pyruvate carrier inhibitor drives stem cell-like memory CAR T cell generation and enhances antitumor efficacy
Abstract
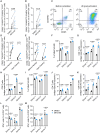
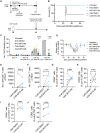
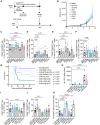
Adoptive cell transfer with chimeric antigen receptor (CAR)-expressing T cells can induce remarkable complete responses in cancer patients. Therapeutic success has been correlated with central and stem cell-like memory T cell subsets in the infusion product, which are better able to drive efficient CAR T cell in vivo expansion and long-term persistence. We previously reported that inhibition of the mitochondrial pyruvate carrier (MPC) during mouse CAR T cell culture induces a memory phenotype and enhances antitumor efficacy against melanoma. Here, we use a novel MPC inhibitor, MITO-66, which robustly induces a stem cell-like memory phenotype in CD19-CAR T cells generated from healthy donors and patients with relapsed/refractory B cell malignancies. MITO-66-conditioned CAR T cells were superior in controlling human pre-B cell acute lymphoblastic leukemia in mice. Following adoptive cell transfer, MITO-66-conditioned CAR T cells maintained a memory phenotype and protected cured mice against tumor rechallenge. Furthermore, in an in vivo B cell leukemia stress model, CD19-CAR T cells generated in the presence of MITO-66 largely outperformed clinical-stage AKT and PI-3Kδ inhibitors. Thus, we provide compelling preclinical evidence that MPC inhibition with MITO-66 during CAR T cell manufacturing dramatically enhances their antitumor efficacy, thereby paving the way to clinical translation.
Keywords: CAR T cell manufacture; CAR T cell therapy; MT: Regular Issue; immunometabolism; memory T cell differentiation; mitochondrial pyruvate carrier.
© 2024 The Author(s).
Conflict of interest statement
M.W. and P.R. are inventors on a patent on MPC inhibition for memory T cell development. M.W. and J.-C.M. are part of the management team at MPC Therapeutics, a Geneva-based start-up that develops MITO-66. D.M. and P.R. are members of the scientific advisory board at MPC Therapeutics. D.M. is an inventor of patents related to CAR-T cell therapy, filed by the University of Pennsylvania, the Istituto Oncologico della Svizzera Italiana (IOSI), and the University of Geneva. D.M. is a scientific cofounder of Cellula Therapeutics SA.
Figures




References
-
- Rohaan M.W., Borch T.H., van den Berg J.H., Met Ö., Kessels R., Geukes Foppen M.H., Stoltenborg Granhøj J., Nuijen B., Nijenhuis C., Jedema I., et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2022;387:2113–2125. doi: 10.1056/NEJMoa2210233. - DOI - PubMed
-
- Morgan R.A., Dudley M.E., Wunderlich J.R., Hughes M.S., Yang J.C., Sherry R.M., Royal R.E., Topalian S.L., Kammula U.S., Restifo N.P., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. - DOI - PMC - PubMed
-
- Fraietta J.A., Lacey S.F., Orlando E.J., Pruteanu-Malinici I., Gohil M., Lundh S., Boesteanu A.C., Wang Y., O'Connor R.S., Hwang W.T., et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
