Mycophenolate mofetil after tacrolimus for refractory clinically amyopathic dermatomyositis: a case report
- PMID: 39559735
- PMCID: PMC11570807
- DOI: 10.3389/fphar.2024.1472667
Mycophenolate mofetil after tacrolimus for refractory clinically amyopathic dermatomyositis: a case report
Abstract
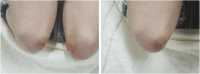
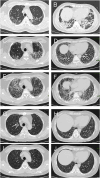
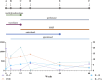
Dermatomyositis (DM) positive for anti-melanoma differentiation-associated gene 5 (MDA5) antibodies, mainly when linked with rapidly progressive interstitial lung disease (RP-ILD), is considered a refractory disease. Our report describes a critical case of clinically amyopathic dermatomyositis (CADM) with RP-ILD that tested positive for both anti-MDA5 and anti-Ro-52 antibodies. The patient showed a limited response to a combined therapy regimen of prednisone, iguratimod, and tacrolimus. However, after adjunct therapy with mycophenolate mofetil (MMF), the patient's condition was controlled, his serum KL-6 levels decreased, and anti-MDA5 antibodies became negative. During the 68-week follow-up, the patient's condition remained stable, with a satisfactory quality of life. This report also discusses the potential role of inflammatory cytokines in the pathophysiology of CADM and RP-ILD. Further research is required to confirm these results and investigate the application of MMF in maintenance therapy for CADM-associated RP-ILD.
Keywords: anti-MDA5-associated dermatomyositis; case report; clinically amyopathic dermatomyositis (CADM); mycophenolate mofetil; rapidly progressive interstitial lung disease.
Copyright © 2024 Ling, Su, Guo, Qiu, Liu, Xiao, Xiao, Yang, Zhang and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

