JAK inhibitors: an evidence-based choice of the most appropriate molecule
- PMID: 39559737
- PMCID: PMC11570808
- DOI: 10.3389/fphar.2024.1494901
JAK inhibitors: an evidence-based choice of the most appropriate molecule
Abstract
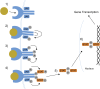
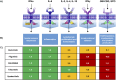
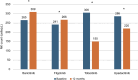
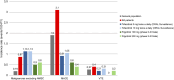
Janus kinase inhibitors (JAKis) represent a fundamental therapeutic tool for the treatment of patients with immune-mediated inflammatory diseases. Although JAKis are often considered a homogeneous class of drugs whose members are thought to be largely interchangeable, there are significant differences in their efficacy and safety profiles. This narrative review analyzes the pharmacokinetic and pharmacodynamic differences among JAKIs, highlighting their clinical relevance based on the most recent available evidence. The article aims to provide rheumatologists, gastroenterologists and dermatologists with practical guidance for choosing the most appropriate JAKi for each patient, given the lack of evidence-based recommendations on this topic, to improve clinical outcomes. Due to its preferential action on JAK1, intestinal metabolization and proven absence of impact on male fertility, filgotinib may be characterized by an improved benefit/risk ratio compared with other less selective JAKis.
Keywords: JAK inhibitors; efficacy; pharmacodynamic; pharmacokinetic; safety.
Copyright © 2024 Antonioli, Armuzzi, Fantini and Fornai.
Conflict of interest statement
AA declares he received consulting/advisory board fees from AbbVie, Alfa-Sigma, Astra Zeneca, Biogen, Bristol-Myers Squibb, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Giuliani, Janssen, Lionhealth, MSD, Nestlé, Pfizer, Roche, Sanofi, Samsung Bioepis, Sandoz, Takeda, Tillots Pharma; speaker’s fees from AbbVie, AG Pharma, Biogen, Bristol-Myers Squibb, Celltrion, Eli-Lilly, Ferring, Galapagos, Janssen, Lionealth, MSD, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, Teva Pharmaceuticals; and research grants from MSD, Takeda, Pfizer, Biogen. MFa has acted as a consultant for: AbbVie, Celgene, Celltrion, Gilead, Pfizer, MSD, Bristol-Meyer, Takeda, Janssen-Cilag, Roche, Galapagos, Biogen; Sandoz, Eli-Lilly, Teva, Giuliani, he has received financial support for research from Janssen-Cilag, Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Anderson K., Nelson C. H., Gong Q., Alani M., Tarnowski T., Othman A. A. (2022). Assessment of the effect of filgotinib on the pharmacokinetics of atorvastatin, pravastatin, and rosuvastatin in healthy adult participants. Clin. Pharmacol. Drug. Dev. 11, 235–245. 10.1002/cpdd.1015 - DOI - PMC - PubMed
-
- Askling J., Berglind N., Franzen S., Frisell T., Garwood C., Greenberg J. D., et al. (2016). How comparable are rates of malignancies in patients with rheumatoid arthritis across the world? A comparison of cancer rates, and means to optimise their comparability, in five RA registries. Ann. Rheum. Dis. 75, 1789–1796. 10.1136/annrheumdis-2015-208105 - DOI - PubMed
-
- Bagley C. J., Woodcock J. M., Stomski F. C., Lopez A. F. (1997). The structural and functional basis of cytokine receptor activation: lessons from the common β subunit of the granulocyte-macrophage colony-stimulating factor, interleukin-3 (IL-3), and IL-5 receptors. Blood 89, 1471–1482. 10.1182/blood.v89.5.1471.1471_1471_1482 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

