MOTS-c modulates skeletal muscle function by directly binding and activating CK2
- PMID: 39559755
- PMCID: PMC11570452
- DOI: 10.1016/j.isci.2024.111212
MOTS-c modulates skeletal muscle function by directly binding and activating CK2
Abstract
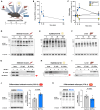
MOTS-c is a mitochondrial microprotein that improves metabolism. Here, we demonstrate CK2 is a direct and functional target of MOTS-c. MOTS-c directly binds to CK2 and activates it in cell-free systems. MOTS-c administration to mice prevented skeletal muscle atrophy and enhanced muscle glucose uptake, which were blunted by suppressing CK2 activity. Interestingly, the effects of MOTS-c are tissue-specific. Systemically administered MOTS-c binds to CK2 in fat and muscle, yet stimulates CK2 activity in muscle while suppressing it in fat by differentially modifying CK2-interacting proteins. Notably, a naturally occurring MOTS-c variant, K14Q MOTS-c, has reduced binding to CK2 and does not activate it or elicit its effects. Male K14Q MOTS-c carriers exhibited a higher risk of sarcopenia and type 2 diabetes (T2D) in an age- and physical-activity-dependent manner, whereas females had an age-specific reduced risk of T2D. Altogether, these findings provide evidence that CK2 is required for MOTS-c effects.
Keywords: Physiology; cell biology.
© 2024 The Author(s).
Conflict of interest statement
Pinchas Cohen is an advisor to and stockholder in CohBar Inc. UCLA has licensed the intellectual property on MOTS-c, on which Pinchas Cohen is listed as an inventor, to CohBar. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures






References
-
- Lee C., Zeng J., Drew B.G., Sallam T., Martin-Montalvo A., Wan J., Kim S.J., Mehta H., Hevener A.L., de Cabo R., Cohen P. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metabol. 2015;21:443–454. doi: 10.1016/j.cmet.2015.02.009. - DOI - PMC - PubMed

