Modeling the Acute Mucosal Toxicity of Fractionated Radiotherapy Combined with the ATM Inhibitor WSD0628
- PMID: 39559836
- PMCID: PMC11791477
- DOI: 10.1158/1535-7163.MCT-24-0664
Modeling the Acute Mucosal Toxicity of Fractionated Radiotherapy Combined with the ATM Inhibitor WSD0628
Abstract
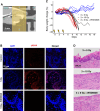
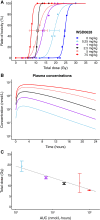
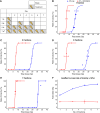
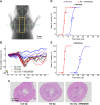
Ataxia Telangiectasia-mutated (ATM) inhibitors are being developed as radiosensitizers to improve the antitumor effects of radiotherapy, but ATM inhibition can also radiosensitize normal tissues. Therefore, understanding the elevated risk of normal tissue toxicities is critical for radiosensitizer development. This study focused on modeling the relationship between acute mucosal toxicity, radiation dose, fractionation schedule, and radiosensitizer exposure. The ATM inhibitor WSD0628 was combined with single or fractionated doses of radiation delivered to the oral cavity or esophagus of Friend Leukemia virus B (FVB) mice. The potentiation by WSD0628 was quantified by a sensitizer enhancement ratio (SER), which describes the changes in radiation tolerance for radiation combined with WSD0628 relative to radiation-only regimens. WSD0628 profoundly enhanced radiation-induced acute oral and esophageal toxicities. For oral mucosal toxicity, the enhancement by WSD0628 with 3 fractions of radiation resulted in an SER ranging from 1.3 (0.25 mg/kg) to 3.1 (7.5 mg/kg). For the 7.5 mg/kg combination, the SER increased with increasing number of fractions from 2.2 (1 fraction) to 4.3 (7 fractions) for oral toxicity and from 2.2 (1 fraction) to 3.6 (3 fractions) for esophageal toxicity, which reflects a loss of the normal tissue sparing benefit of fractionated radiation. These findings were used to develop a modified biologically effective dose model to determine alternative radiation schedules with or without WSD0628 that result in similar levels of toxicity. Successful radiosensitizer dose escalation to a maximally effective therapeutic dose will require careful deliberation of tumor site and reduction of radiation dose volume limits for organs at risk.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J.E. Eckel-Passow reports grants from the NIH during the conduct of the study. W. Zhong reports nonfinancial support from Wayshine Biopharm during the conduct of the study and personal fees and nonfinancial support outside the submitted work; in addition, W. Zhong has a patent for US11919899 issued and a patent for US18841286 pending. J.N. Sarkaria reports grants from Wayshine Biopharm during the conduct of the study and grants from Bayer, Black Diamond, Karyopharm, Boston Scientific, Wugen, Rain Therapeutics, Sumitomo Dainippon Pharma Oncology, AbbVie, SK Biopharmaceuticals, Boehringer Ingelheim, AstraZeneca, ABL Bio, Inhibrx, Otomagnetics, grants from Reglagene, and Breakpoint Therapeutics outside the submitted work. No disclosures were reported by the other authors.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

