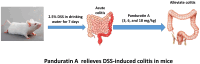
Panduratin A mitigates inflammation and oxidative stress in DSS-induced colitis mice model
- PMID: 39559852
- PMCID: PMC11581177
- DOI: 10.1080/20565623.2024.2428129
Panduratin A mitigates inflammation and oxidative stress in DSS-induced colitis mice model
Abstract
Aim: This study explored Panduratin A's protective effects against DSS-induced colitis in mice, focusing on reducing inflammation and oxidative stress in the colon.
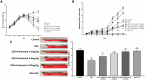
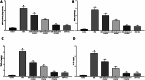
Methods: Mice were treated with dextran sodium sulfate (DSS) and Panduratin A (3, 6, 18 mg/kg), and changes in body weight, colon length, Disease Activity Index (DAI), histopathology, inflammation markers including tumor necrosis factor- α (TNF-α), Interleukin-1 β (IL-1β), Myeloperoxidase (MPO), and oxidative stress, Malondialdehyde (MDA) were evaluated.
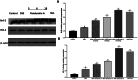
Results: Panduratin A significantly reversed DSS-induced symptoms, including body weight loss, colonic length shortening, and DAI increase, while reducing histopathological damage. It lowered inflammatory markers and oxidative stress, suppressed NF-κB activation, and enhanced Nrf2 and HO-1 expression.
Conclusion: Panduratin A shows promise as a colitis treatment, warranting further research for broader clinical application.
Keywords: DSS-induced colitis; Panduratin A; anti-inflammatory; oxidative stress; ulcerative colitis.
Plain language summary
In this study, we looked at how a natural compound called Panduratin A might help fight a common type of bowel inflammation called colitis in mice. This inflammation can cause pain and other health problems. We treated mice with a substance that causes colitis, then gave some of them Panduratin A to see if it would help. We watched how their weight changed, how long their colons were, and how much inflammation they had. Our results were promising: the mice treated with Panduratin A were healthier than those that weren’t. They lost less weight, had longer colons, and showed fewer signs of inflammation. This suggests that Panduratin A could be a new way to protect against bowel inflammation. More studies are needed to see if this treatment could work in people too.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
