NSAID Allergy Labels Associated With Mortality and Cardiovascular Outcomes in Stroke
- PMID: 39559853
- PMCID: PMC11812653
- DOI: 10.1161/STROKEAHA.124.047921
NSAID Allergy Labels Associated With Mortality and Cardiovascular Outcomes in Stroke
Abstract
Background: Mislabeled drug allergy can restrict future prescriptions and medication use, but its prevalence and impact among patients with stroke remain unknown. This study investigated the prevalence of the most commonly labeled drug allergies, their accuracy, and their impact among patients with stroke.
Methods: In this combined longitudinal and cross-sectional study, we compared the prevalence of allergy labels among the general population and patients with ischemic stroke between 2008 and 2014 from electronic health care records in Hong Kong. Outcomes between patients with stroke with or without the most prevalent labels (ie, NSAID) were compared. Rate of mislabeled NSAID allergy was confirmed by provocation testing.
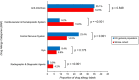
Results: Compared with the general population (n=702 966), patients with stroke had more labels (n=235) to cardiovascular and hematopoietic system (prevalence, 19.5% versus 9.2%; odds ratio [OR], 2.4 [95% CI, 1.74-3.32]; P<0.001) and radiographic and diagnostic agents (prevalence, 4.2% versus 0.9%; OR, 4.82 [95% CI, 2.56-9.08]; P<0.001). The most common labels were to NSAID (prevalence, 1.8%). Patients with NSAID allergy labels were significantly less likely to be prescribed aspirin after acute stroke (OR, 0.24 [95% CI, 0.09-0.60]; P=0.003) and on follow-up (OR, 0.22 [95% CI, 0.08-0.56]; P=0.002). The median duration of follow-up was 6.7 years (6499±2.49 patient-years). Patients with stroke with NSAID allergy labels also experienced significantly higher mortality (OR, 7.44 [95% CI, 2.44-23.18]; P<0.001), peripheral vascular disease (OR, 9.35 [95% CI, 1.95-44.86]; P=0.005), and major adverse cardiovascular events (OR, 6.09 [95% CI, 2.00-18.58]; P=0.001) in the poststroke period. Patients with NSAID allergy labels (who remained alive and could consent) were referred for allergist assessment and offered drug provocation testing. The majority (80%; 4/5) had negative provocation tests and were delabeled.
Conclusions: NSAID allergy labels were significantly more prevalent among patients with stroke, associated with excessive mortality, peripheral vascular disease, and major adverse cardiovascular events. Given the high rate of mislabeled allergies, multidisciplinary neuro-allergy interventions could have the potential to improve patient outcomes.
Keywords: Hong Kong; cardiovascular diseases; mortality; peripheral vascular diseases; stroke.
Conflict of interest statement
None.
Figures
References
-
- Chong CJ, Choo KJL, Ong KY, Tan V, Khoo JBN, Murthee KG, Hanif IM, Naing CS, Lee HY. Improving drug allergy label accuracy by supervised safety- and protocol-driven evaluation. Ann Acad Med Singap. 2022;51:677–685. doi: 10.47102/annals-acadmedsg.2022118 - PubMed
-
- Sacco KA, Bates A, Brigham TJ, Imam JS, Burton MC. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288–1296. doi: 10.1111/all.13168 - PubMed
-
- Siew LQC, Li PH, Watts TJ, Thomas I, Ue KL, Caballero MR, Rutkowski K, Till SJ, Pillai P, Haque R. Identifying low-risk beta-lactam allergy patients in a UK tertiary centre. J Allergy Clin Immunol Pract. 2019;7:2173–2181.e1. doi: 10.1016/j.jaip.2019.03.015 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical