Informing Alzheimer's Biomarker Communication: Concerns and Understanding of Cognitively Unimpaired Adults During Amyloid Results Disclosure
- PMID: 39559870
- PMCID: PMC11573811
- DOI: 10.14283/jpad.2024.151
Informing Alzheimer's Biomarker Communication: Concerns and Understanding of Cognitively Unimpaired Adults During Amyloid Results Disclosure
Abstract
Background: Biomarker results are increasingly disclosed in research and clinical settings, but less is known about how individuals interpret their results or concerns raised during the disclosure visit that may need to be addressed by clinicians to ensure appropriate disclosure.
Methods: Fifty-two cognitively unimpaired older adults aged 65 to 89 years old from the Wisconsin Registry for Alzheimer's Prevention, who had undergone an amyloid PET scan in the previous 18 months, were enrolled in the disclosure substudy. After ensuring psychological readiness, trained study clinicians disclosed amyloid PET results using a structured protocol. We assessed participants' level of understanding, concerns, and the perceived personal significance of their biomarker results during the disclosure visit through a series of question prompts in real-time.
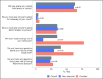
Results: Thirty-four received a non-elevated amyloid result and 18 received an elevated result. The average age was 72.2 years (range 65-81); most were women (64%) and non-Hispanic White (92%). Participants understood their results (98%), and both non-elevated and elevated groups provided similar responses around topics of sharing with others, privacy, accuracy of testing, and risk. Participants with elevated results were significantly more likely than those with non-elevated results to want to change their lifestyle (78% vs 12%, p=<0.01) and have questions about their results (61% vs 30%, p=0.05). Participants interpreted the personal significance of results in terms of several themes relating to individual risk status, emotional impact, whether the result was expected, and prevention/planning.
Conclusion: Results show that participants understand their biomarker results, and have a number of concerns during the disclosure process that clinical and research protocols could address. en These findings could be important considerations as effective processes are developed for widespread biomarker disclosure in clinical and research settings.
Keywords: AD biomarkers; Alzheimer’s Disease; Amyloid; biomarker disclosure; dementia education; ethics and communication; preclinical AD.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
