Phase 1 Studies of the Anti-Tau Monoclonal Antibody JNJ-63733657 in Healthy Participants and Participants with Alzheimer's Disease
- PMID: 39559872
- PMCID: PMC11573813
- DOI: 10.14283/jpad.2024.163
Phase 1 Studies of the Anti-Tau Monoclonal Antibody JNJ-63733657 in Healthy Participants and Participants with Alzheimer's Disease
Abstract
Background: JNJ-63733657 (posdinemab) is a humanized IgG1/kappa monoclonal anti-phospho tau antibody that binds with high affinity to phosphorylated amino acid 217 (pT217) in the proline-rich domain. The parent molecule, PT3, was raised against Alzheimer's disease brain purified paired helical filament, and preclinical studies with the humanized version, JNJ-63733657, have demonstrated reductions in tau seeding. The results of the first-in-human clinical trial of JNJ-63733657 and a separate single ascending dose study in Japanese participants are presented.
Objectives: To evaluate the safety and tolerability, pharmacokinetics, immunogenicity, and pharmacodynamics of JNJ-63733657 after single and multiple intravenous dose administrations in healthy participants and participants with prodromal or mild Alzheimer's disease.
Design: A two part first-in-human, phase 1, randomized, double-blind, placebo-controlled trial: Single ascending dose (Part 1) and multiple ascending dose (Part 2). And a phase 1, randomized, double-blind, placebo-controlled single ascending dose trial in healthy Japanese participants.
Setting: 7 sites in Belgium, Netherlands, Spain, and Germany; 1 site in Japan.
Participants: A total of 40 healthy participants aged 55-75 were enrolled in Part 1 of the first-in-human study; a total of 16 healthy participants and 13 participants with prodromal or mild AD aged 55-80 years were enrolled in Part 2. In the Japanese trial, a total of 24 participants aged 55-75 were enrolled.
Intervention: In Part 1, single doses of 1, 3, 10, 30, or 60 mg/kg of JNJ-63733657 or placebo were administered to healthy participants. In Part 2, two dose levels of JNJ-63733657 (5 mg/kg or 50 mg/kg) or placebo were evaluated in healthy participants, and 2 dose levels (15 mg/kg or 30 mg/kg) or placebo were evaluated in participants with Alzheimer's disease; doses were administered on Days 1, 29, and 57. In the Japanese trial, single doses of 3, 15, or 60 mg/kg of JNJ-63733675 or placebo were administered. All doses were administered intravenously.
Measurements: Safety assessments, serum and cerebrospinal fluid pharmacokinetic parameters, immunogenicity, and cerebrospinal fluid pharmacodynamic changes in free and total p217+tau, total tau, and p181tau were evaluated.
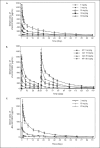
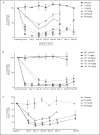
Results: JNJ-63733657 was generally safe and well-tolerated in healthy participants and participants with Alzheimer's disease. In healthy participants and participants with Alzheimer's disease, JNJ-63733657 demonstrated linear PK, and serum Cmax and AUC were approximately dose proportional following single and multiple doses. Dose-dependent reductions in free and total p217+tau in cerebrospinal fluid were observed. No changes in total tau or p181tau were observed in healthy participants whereas Alzheimer's disease participants showed decreases in these tau species following administration of JNJ-63733657.
Conclusion: In these Phase 1 trials, no safety or tolerability concerns were identified, and dose dependent reductions in p217+tau in the cerebrospinal fluid were demonstrated following administration of JNJ-63733657. The safety and biomarker profiles support the continued investigation of this compound for the slowing of disease progression in Alzheimer's disease.
Keywords: monoclonal antibody; Alzheimer’s disease; Phase 1 trial; p217+tau; tau.
Conflict of interest statement
All authors are current or former employees of Janssen Pharmaceuticals and may hold stock or stock options in Johnson and Johnson
Figures
References
-
- A Donanemab (LY3002813) Prevention Study in Participants With Alzheimer’s Disease (TRAILBLAZER-ALZ 3), Eli Lilly and Company, https://clinicaltrials.gov/, accessed 2 April 2024.
-
- AHEAD 3–45 Study: A Study to Evaluate Efficacy and Safety of Treatment With Lecanemab in Participants With Preclinical Alzheimer’s Disease and Elevated Amyloid and Also in Participants With Early Preclinical Alzheimer’s Disease and Intermediate Amyloid, Eisai Inc, https://clinicaltrials.gov, accessed 2 April 2024.
-
- Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J Prev Alzheimers Dis 2022; 9:197–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
