Roles of TREM2 in the Pathological Mechanism and the Therapeutic Strategies of Alzheimer's Disease
- PMID: 39559879
- PMCID: PMC11573818
- DOI: 10.14283/jpad.2024.164
Roles of TREM2 in the Pathological Mechanism and the Therapeutic Strategies of Alzheimer's Disease
Abstract
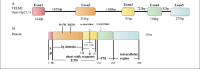
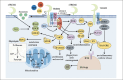
Alzheimer's disease (AD) is an age-related degenerative disease, which is characteristic by the deposition of senile plaques (SP) outside the cells, the neurofibrillary tangles (NFTs) inside the neurons, and the loss of synapse and neurons. Neuroinflammation may play an important role in the pathogenesis of AD. Microglia are the immune cells in the central nervous system. However, microglia might become disease-related microglia (DAMs) when stimulated by the external environment. DAMs have been shown to be involved in a series of events of AD development including Aβ accumulation and tau phosphorylation. The triggering receptor expressed on myeloid cells 2 (TREM2) is a transmembrane receptor that is mainly expressed by microglia in the central nervous system (CNS). TREM2 plays an important role in the physiological function of microglia, and the dyshomeostasis of TREM2 is related to the development of late-onset AD. This article summarized the latest advances in TREM2 biology and its impact on the roles of microglia in AD development, with a particular emphasis on the structure, ligands, signal transduction, and the agonistic antibodies of TREM2 for AD treatment. We further discussed the survival, migration, phagocytosis, inflammation, and cellular metabolism of microglia, as well as the role of sTREM2 in neuroprotection and as a biomarker for AD. It provides a reference for further research on the molecular mechanism of microglial TREM2 in the occurrence and development of AD and on the therapeutic strategies targeted on the microglial TREM2.
Keywords: Alzheimer’s disease (AD); Aβ; microglia; monoclonal antibody; triggering receptor expressed on myeloid cells 2 (TREM2).
Conflict of interest statement
The authors have no conflict of interest to report.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
