Intermittent Hypoxic-Hyperoxic Training During Inpatient Rehabilitation Improves Exercise Capacity and Functional Outcome in Patients With Long Covid: Results of a Controlled Clinical Pilot Trial
- PMID: 39559920
- PMCID: PMC11634465
- DOI: 10.1002/jcsm.13628
Intermittent Hypoxic-Hyperoxic Training During Inpatient Rehabilitation Improves Exercise Capacity and Functional Outcome in Patients With Long Covid: Results of a Controlled Clinical Pilot Trial
Abstract
Introduction: Long COVID-19 illness is a severely disabling disease with shortness of breath, weakness and fatigue as leading symptoms, resulting in poor quality of life and substantial delay in return to work. No specific respiratory therapy has been validated for patients with long COVID. The intermittent hypoxia-hyperoxia training (IHHT) is a respiratory therapeutic modality to improve exercise performance via controlled respiratory conditioning. The purpose of the present study is to investigate the therapeutic effect of IHHT on functional and symptomatic recovery of patients with long COVID syndrome.
Methods: A prospective, controlled, open-treatment interventional study was conducted in patients with long COVID who were admitted to an inpatient rehabilitation programme. Patients were assigned nonrandomized to receive IHHT in addition to the standardized rehabilitation programme (IHHT group) or standard rehabilitation alone (control group). The IHHT group received supervised sessions of intermittent hypoxic (10-12% O2) and hyperoxic (30-35% O2) breathing three times per week throughout the rehabilitation period. Primary endpoint was improved walking distance in a 6-min walk test (6MWT) between study groups. Secondary endpoints were change in stair climbing power, dyspnoea (Borg dyspnoea Scale), fatigue assessment scale (FAS) and change in health-related quality of life (HRQoL) assessed by patient global assessment (PGA), EQ-5D analogue scale and the MEDIAN Corona Recovery Score (MCRS). Further assessments included maximum handgrip strength, nine hole peg test, timed up-and-go, respiratory function and functional ambulation category (FAC), serum analyses and safety of the intervention.
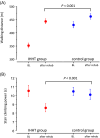
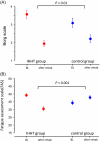
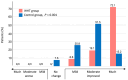
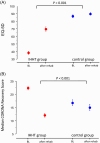
Results: A total of 145 patients were included in the study (74% female, mean age 53 ± 12 years) and assigned to IHHT (n = 70) or standard care (n = 75). The 6MWT distance improved 2.8-fold in the IHHT group compared to the control group (91.7 ± 50.1 m vs. 32.6 ± 54.2 m, ANCOVA p < 0.001). Stair climbing power improved 3.7-fold in the IHHT group compared to controls (-1.91 ± 2.23 s vs. -0.51 ± 1.93 s, p < 0.001). Secondary endpoints on dyspnoea, fatigue and HRQoL (PGA, EQ-5D and MCRS) improved significantly in the IHHT group compared to controls. The IHHT group exhibited a significant decrease in blood pressure, heart rate and increase in haemoglobin levels that was not observed in the control group. No adverse events were observed.
Conclusion: Respiratory treatment with IHHT in addition to a multidisciplinary rehabilitation programme improves functional capacity, symptomatic status and quality of life in patients with disabling long COVID. IHHT has been demonstrated to be safe, well tolerated and feasible to be integrated in an inpatient rehabilitation programme to improve outcome in long COVID.
Keywords: 6 min walking test; hypoxia; long COVID; rehabilitation; training.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
W. Doehner reports consulting fees and speaker honoraria from Bayer, Boehringer Ingelheim, Boston Scientific, Cardiomatics, Aimediq, Medtronic, Vifor Pharma, travel support from Pharmacosmos and research support to the Institute from EU (Horizon2020), German Ministry of Education and Research, German Center for Cardiovascular Research, German Pension Insurance (regional Mitteldeutschland) Boehringer Ingelheim, Vifor Pharma. C. Altmann reports consulting fees, speaker honoraria and research support from Pfizer, Amgen, Amarin, Novartis. P. Schüller declares consulting fees, speaker honoraria and research support from Pfizer, Amgen, Amarin and Novartis. A. Shafieesabet, B. Alimi, J. Muhar and J. Springer report no conflict of interest.
Figures
References
-
- NICE , “Guideline on Management of Long COVID,” https://www.nice.org.uk/guidance/NG188.
-
- Rahmati M., Udeh R., Yon D. K., et al., “A Systematic Review and Meta‐Analysis of Long‐Term Sequelae of COVID‐19 2‐Year After SARS‐CoV‐2 Infection: A Call to Action for Neurological, Physical, and Psychological Sciences,” Journal of Medical Virology 95 (2023): e28852, 10.1002/jmv.28852. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

