Homocysteine, neurodegenerative biomarkers, and APOE ε4 in neurodegenerative diseases
- PMID: 39559926
- PMCID: PMC11775453
- DOI: 10.1002/alz.14376
Homocysteine, neurodegenerative biomarkers, and APOE ε4 in neurodegenerative diseases
Abstract
Introduction: Elevated plasma homocysteine (Hcy) is associated with an increased risk of developing neurodegenerative diseases; however, its relationship with the apolipoprotein E (APOE) ε4 allele has not been well characterized.
Methods: Participants clinically diagnosed with Alzheimer's disease or mild cognitive impairment (AD/MCI), frontotemporal dementia, Parkinson's disease, or cerebrovascular disease were stratified by the presence of the APOE ε4 allele. Volumetric magnetic resonance imaging, plasma amyloid/tau/neurodegeneration biomarkers, and cognitive performance were quantified.
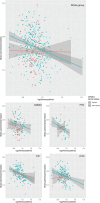
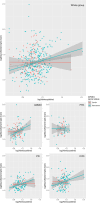
Results: Across all diagnostic groups, Hcy was associated with lower brain parenchymal fraction and greater neurofilament light chain in APOE ε4 non-carriers only. In AD/MCI, Hcy was associated with phosphorylated tau 217 in APOE ε4 non-carriers, but not in carriers. Exploratory analyses revealed interactions between Hcy and APOE ε4 on memory and visuospatial function.
Discussion: Hcy may contribute to neurodegeneration depending on the presence of the APOE ε4 allele and specific disease processes. Trials on vitamin B12 supplementation may consider stratifying by APOE genotype. Highlights Homocysteine (Hcy) was associated with neurodegenerative biomarkers across disease groups. Relationships with Hcy were predominantly found in apolipoprotein E (APOE) ε4 non-carriers. In Alzheimer's disease, associations between Hcy and phosphorylated tau 217 were found in APOE ε4 non-carriers only. Significant interactions existed between Hcy and APOE ε4 status on cognition.
Keywords: Alzheimer's disease; Parkinson's disease; apolipoprotein E; biomarkers; cerebrovascular disease; dementia; frontotemporal dementia; homocysteine; mild cognitive impairment.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
All other authors report no relevant conflicts of interest. Author disclosures are available in supporting information.
Figures

References
MeSH terms
Substances
Grants and funding
- Ontario Brain Institute
- Baycrest Foundation
- Bruyère Research Institute
- Centre for Addiction and Mental Health Foundation
- London Health Sciences Foundation
- McMaster University Faculty of Health Sciences
- Ottawa Brain and Mind Research Institute
- Queen's University Faculty of Health Sciences
- Thunder Bay Regional Health Sciences Centre
- University of Ottawa Faculty of Medicine
- Windsor/Essex County ALS Association
- Temerty Family Foundation
- ER21-16-141/Ontario Ministry of Colleges and Universities
- CRC-2020-00353/Canada Research Chairs Program
- 202111FBD-476226/Canada Graduate Scholarships
- 2023-00356/Swedish Research Council
- 2022-01018/Swedish Research Council
- 2019-02397/Swedish Research Council
- 101053962/European Union's Horizon Europe research and innovation programme
- ALFGBG-71320/Swedish State Support for Clinical Research
- 201809-2016862/Alzheimer Drug Discovery Foundation
- AD Strategic Fund
- Bluefield Project
- Olav Thon Foundation
- Erling-Persson Family Foundation
- Stiftelsen för Gamla Tjänarinnor
- FO2022-0270/Hjärnfonden
- 860197 (MIRIADE)/European Union's Horizon 2020 research and innovation
- JPND2021-00694/European Union Joint Programme - Neurodegenerative Disease Research
- National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre
- UKDRI-1003/UK Dementia Research Institute
- ADSF-21-831376-C/ALZ/Alzheimer's Association/United States
- ADSF-21-831381-C/ALZ/Alzheimer's Association/United States
- ADSF-21-831377-C/ALZ/Alzheimer's Association/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

