Longitudinal multimodal profiling of IDH-wildtype glioblastoma reveals the molecular evolution and cellular phenotypes underlying prognostically different treatment responses
- PMID: 39560080
- PMCID: PMC11726253
- DOI: 10.1093/neuonc/noae214
Longitudinal multimodal profiling of IDH-wildtype glioblastoma reveals the molecular evolution and cellular phenotypes underlying prognostically different treatment responses
Abstract
Background: Despite recent advances in the biology of IDH-wildtype glioblastoma, it remains a devastating disease with median survival of less than 2 years. However, the molecular underpinnings of the heterogeneous response to the current standard-of-care treatment regimen consisting of maximal safe resection, adjuvant radiation, and chemotherapy with temozolomide remain unknown.
Methods: Comprehensive histopathologic, genomic, and epigenomic evaluation of paired initial and recurrent glioblastoma specimens from 106 patients was performed to investigate the molecular evolution and cellular phenotypes underlying differential treatment responses.
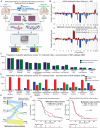
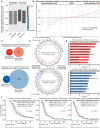
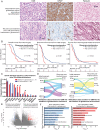
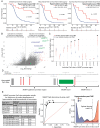
Results: While TERT promoter mutation and CDKN2A homozygous deletion were early events during gliomagenesis shared by initial and recurrent tumors, most other recurrent genetic alterations (eg, EGFR, PTEN, and NF1) were commonly private to initial or recurrent tumors indicating acquisition later during clonal evolution. Furthermore, glioblastomas exhibited heterogeneous epigenomic evolution with subsets becoming more globally hypermethylated, hypomethylated, or remaining stable. Glioblastoma that underwent sarcomatous transformation had shorter interval to recurrence and were significantly enriched in NF1, TP53, and RB1 alterations and the mesenchymal epigenetic class. Patients who developed somatic hypermutation following temozolomide treatment had significantly longer interval to disease recurrence and prolonged overall survival, and increased methylation at 4 specific CpG sites in the promoter region of MGMT was significantly associated with this development of hypermutation. Finally, an epigenomic evolution signature incorporating change in DNA methylation levels across 347 critical CpG sites was developed that significantly correlated with clinical outcomes.
Conclusions: Glioblastoma undergoes heterogeneous genetic, epigenetic, and cellular evolution that underlies prognostically different treatment responses.
Keywords: DNA methylation; glioblastoma; gliosarcoma; molecular neuropathology; temozolomide-induced hypermutation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
Jennie Taylor, John de Groot, Annette Molinaro, Joseph Costello, Aaron Diaz, Susan Chang, and Mitchel Berger are members of the editorial board of
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–996. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

