Statin-associated regulation of hepatic PNPLA3 in patients without known liver disease
- PMID: 39560367
- PMCID: PMC11636427
- DOI: 10.1111/joim.20032
Statin-associated regulation of hepatic PNPLA3 in patients without known liver disease
Abstract
Background and objectives: Statins are used for metabolic dysfunction-associated steatotic liver disease (MASLD) (NAFLD) treatment, but their role in this context is unclear. Genetic variants of patatin-like phospholipase domain containing 3 (PNPLA3) are associated with MASLD susceptibility and statin treatment efficacy. Access to liver biopsies before established MASLD is limited, and statins and PNPLA3 in early liver steatosis are thus difficult to study.
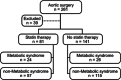
Methods: Liver biopsies were collected from 261 patients without known liver disease at surgery and stratified based on statin use and criteria for the metabolic syndrome (MS). Genotypes and transcript levels were measured using Illumina and Affymetrix arrays, and metabolic and lipoprotein profiles by clinical assays. Statin effects on PNPLA3, de novo lipogenesis (DNL), and lipid accumulation were further studied in vitro.
Results: The PNPLA3I148M genetic variant was associated with significantly lower hepatic levels of cholesterol synthesis-associated transcripts. Patients with MS had significantly higher hepatic levels of MASLD and lipogenesis-associated transcripts than non-MS patients. Patients with MS on statin therapy had significantly higher hepatic levels of PNPLA3, acetyl-CoA carboxylase alpha, and ATP citrate lyase, and statin use was associated with higher plasma fasting glucose, insulin, and HbA1c. Exposure of hepatocyte-like HepG2 cells to atorvastatin promoted intracellular accumulation of triglycerides and lipogenesis-associated transcripts. Atorvastatin-exposure of HepG2, sterol O-acyltransferase (SOAT) 2-only-HepG2, primary human hepatic stellate, and hepatic stellate cell-like LX2 cells significantly increased levels of PNPLA3 and SREBF2-target genes, whereas knockdown of SREBF2 attenuated this effect.
Conclusions: Collectively, these observations suggest statin-associated regulation of PNPLA3 and DNL in liver. The potential interaction between PNPLA3 genotype and metabolic status should be considered in future studies in the context of statin therapy for MASLD.
Keywords: MASLD; NAFLD; lipogenesis; metabolic syndrome.
© 2024 The Author(s). Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
OA, MP, and PP are cofounders and co‐owners of Lipoprotein Research Stockholm AB. PSO is a shareholder of Emune AB.
Figures




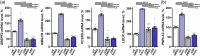


References
-
- Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. - PubMed
-
- Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43:1–23. - PubMed
-
- Almeda‐Valdes P, Aguilar‐Olivos N, Uribe M, Mendez‐Sanchez N. Common features of the metabolic syndrome and nonalcoholic fatty liver disease. Rev Recent Clin Trials. 2014;9:148–158. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

