Tapinarof, an Aryl Hydrocarbon Receptor Ligand, Mitigates Fibroblast Activation in Thyroid Eye Disease: Implications for Novel Therapy
- PMID: 39560627
- PMCID: PMC11578154
- DOI: 10.1167/iovs.65.13.40
Tapinarof, an Aryl Hydrocarbon Receptor Ligand, Mitigates Fibroblast Activation in Thyroid Eye Disease: Implications for Novel Therapy
Abstract
Purpose: In thyroid eye disease (TED), activation and proliferation of orbital fibroblasts (OFs) promotes remodeling and causes an increase in the volume of orbital tissue. Platelet-derived growth factors (PDGFs) are elevated in TED and promote OF activation. The aryl hydrocarbon receptor (AHR), a ligand activated nuclear receptor, is important in regulating OF activation. AHR ligands have been evaluated as therapeutic agents for inflammatory diseases. Here, we hypothesize that AHR ligands will block PDGF-induced signaling in TED OFs.
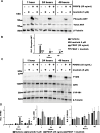
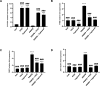
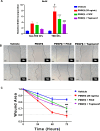
Methods: OFs from both non-TED and TED patients were treated with PDGFβ, with or without the AHR ligands 6-Formylindolo[3,2-b]carbazole (FICZ) or tapinarof. Cell viability was measured by the Alamar Blue assay. Cell proliferation was quantified using the BrdU assay. Cell lysates were collected and analyzed by Western blotting and real-time quantitative PCR (RT-qPCR) to measure PDGF and AHR signaling. Scratch assays were used to measure OF migration.
Results: PDGFβ induced proliferation in TED OFs significantly more than in non-TED OFs. Additionally, PDGFβ increased phosphorylation of AKT and expression of thymidylate synthase (TYMS). PDGFβ dependent proliferation and downstream signaling were attenuated by FICZ or tapinarof. TYMS and other PDGF target genes were upregulated by PDGFβ and reduced by AHR activation. PDGFβ induced TED OF migration while both FICZ and tapinarof diminished this effect.
Conclusions: PDGF signaling led to increased proliferation and activation of TED OFs. Treatment of TED OFs with the AHR ligands, FICZ and tapinarof, mitigated PDGF induced effects. These studies support the concept that AHR and PDGF signaling could form the basis for new TED therapeutics.
Conflict of interest statement
Disclosure:
Figures


References
-
- Diana T, Kahaly GJ. Thyroid stimulating hormone receptor antibodies in thyroid eye disease — methodology and clinical applications. Ophthalmic Plast Reconstr Surg. 2018; 34(4S Suppl 1): S13–S19. - PubMed
-
- Bahn RS. Current insights into the pathogenesis of Graves' ophthalmopathy. Horm Metab Res. 2015; 47: 773–778. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

