Preservation of Murine Whole Eyes With Supplemented UW Cold Storage Solution: Anatomical Considerations
- PMID: 39560629
- PMCID: PMC11578148
- DOI: 10.1167/tvst.13.11.24
Preservation of Murine Whole Eyes With Supplemented UW Cold Storage Solution: Anatomical Considerations
Abstract
Purpose: Retinal ganglion cell (RGC) apoptosis and axon regeneration are the principal obstacles challenging the development of successful whole eye transplantation (WET). The purpose of this study was to create a neuroprotective cocktail that targets early events in the RGC intrinsic apoptotic program to stabilize RGCs in a potential donor eye.
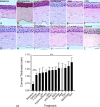
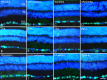
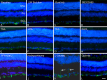
Methods: University of Wisconsin (UW) solution was augmented with supplements known to protect RGCs. Supplements targeted tyrosine kinase signaling, histone deacetylase activity, K+ ion efflux, macroglial stasis, and provided energy support. Modified UW (mUW) solutions with individual supplements were injected into the vitreous of enucleated mouse eyes, which were then stored in cold UW solution for 24 hours. Histopathology, immunostaining of individual retinal cell types, and analysis of cell-specific messenger RNAs (mRNAs) were used to identify supplements that were combined to create optimal mUW solution.
Results: UW and mUW solutions reduced ocular edema and focal ischemia in globes stored in cold storage. Two major issues were noted after cold storage, including retinal detachment and reduction in glial fibrillary acidic protein staining in astrocytes. A combination of supplements resolved both these issues and performed better than the individual supplements alone. Cold storage resulted in a reduction in cell-specific mRNAs, even though it preserved the corresponding protein products.
Conclusions: Eyes treated with optimal mUW solution exhibited preservation of retinal and cellular architecture, but did display a decrease in mRNA levels, suggesting that cold storage induced cellular stasis.
Translational relevance: Application of optimal mUW solution lowers an important barrier to the development of a successful whole eye transplantation procedure.
Conflict of interest statement
Disclosure:
Figures




References
-
- Swain T, McGwin G Jr.. The prevalence of eye injury in the United States, estimates from a meta-analysis. Ophthalmic Epidemiol. 2020; 27: 186–193. - PubMed
-
- Travor MD, Levine ES, Catomeris AJ, et al. .. Disability-adjusted life years resulting from ocular injury among deployed service members, 2001-2020. Ophthalmology. 2024; 131(5): 534–544. (in press). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

