CD2 expressing innate lymphoid and T cells are critical effectors of immunopathogenesis in hidradenitis suppurativa
- PMID: 39560648
- PMCID: PMC11621750
- DOI: 10.1073/pnas.2409274121
CD2 expressing innate lymphoid and T cells are critical effectors of immunopathogenesis in hidradenitis suppurativa
Abstract
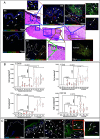
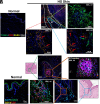
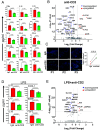
Hidradenitis suppurativa (HS) is a chronic, debilitating inflammatory skin disease with a poorly understood immunopathogenesis. Here, we report that HS lesional skin is characterized by the expansion of innate lymphocytes and T cells expressing CD2, an essential activation receptor and adhesion molecule. Lymphocytes expressing elevated CD2 predominated with unique spatial distribution throughout the epidermis and hypodermis in the HS lesion. CD2+ cells were mainly innate lymphocytes expressing the NK cell marker, CD56, and CD4+ T cells. Importantly, these CD2+ cells interacted with CD58 (LFA3) expressing epidermal keratinocytes and fibroblasts in the hypodermis. Granzyme Abright NKT cells (CD2+CD3+CD56bright) clustered with α-SMA expressing fibroblasts juxtaposed to epithelialized tunnels and fibrotic regions of the hypodermis. Whereas NK cells (CD2+CD56dim) were perforin+, granzymes A+ and B+, and enriched adjacent to hyperplastic follicular epidermis and tunnels of HS showing presence of apoptotic cells. The cytokines IL-12, IL-15, and IL-18, which enhance NK cell maturation and function were significantly elevated in HS. Ex vivo HS skin explant cultures treated with CD2:CD58 interaction-blocking anti-CD2 monoclonal antibody attenuated secretion of inflammatory cytokines/chemokines and suppressed inflammatory gene signature. Additionally, CD2:CD58 blockade altered miRNAs involved in NK/NKT differentiation and/or function. In summary, we show that a cellular network of heterogenous NKT and NK cell populations drives inflammation and is critical in the pathobiology of HS, including tunnel formation and fibrosis. Finally, CD2 blockade is a viable immunotherapeutic approach for the effective management of HS.
Keywords: CD2; CD4 T cell; Hidradenitis suppurativa; NK cell; NKT cell.
Conflict of interest statement
Competing interests statement:E.B. and D.B. are co-founders of ITB-MED. Patent application number: PCT/US2023/022364.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

