The P38MAPK Pathway Mediates the Destruction of the Blood-Brain Barrier in Anti-NMDAR Encephalitis Mice
- PMID: 39560818
- PMCID: PMC11576659
- DOI: 10.1007/s11064-024-04270-1
The P38MAPK Pathway Mediates the Destruction of the Blood-Brain Barrier in Anti-NMDAR Encephalitis Mice
Abstract
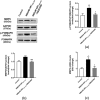
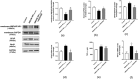
The clinical manifestations of anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis may be closely related to the integrity of the blood-brain barrier (BBB). The P38 mitogen-activated protein kinase (P38MAPK) pathway plays a protective role in neurodegenerative diseases. However, whether the P38MAPK pathway is involved in the underlying mechanism of tight junction (TJ) protein disruption and neuronal damage has not been elucidated. Therefore, in this study, a mouse model of anti-NMDAR encephalitis was established by active immunization with NMDAR NR1356-385 peptides. The critical pathways of P38MAPK were screened by interaction network and co-enrichment analysis. The role of P38MAPK pathways was investigated by the injection of P38MAPK inhibitor SB203580 (10 mg/kg, i.p.). Compared with the control group, the expression of occludin and zonula occludens (ZO)-1 in NMDAR NR1356-385 group mice was downregulated, and the structure and function of BBB were damaged. However, after the intervention of SB203580, the activation of the P38MAPK was inhibited, the expression of matrix metalloproteinase 9 (MMP9) was reduced, and the function of BBB was improved. Meanwhile, inhibiting the P38MAPK pathway reversed the degradation of NMDAR NR1, while reducing the expression of the glial fibrillary acidic protein (GFAP) and pro-inflammatory factor tumor necrosis factor (TNF-α). It also relieved the damage of neuron-specific nucleus (NeuN), thus alleviating psychobehavioral symptoms. In conclusion, our results suggested that the P38MAPK pathway is involved in BBB destruction and neurobehavioral change in mice with anti-NMDAR encephalitis. Targeting the P38MAPK pathway may be a promising option for the treatment of anti-NMDAR encephalitis.
Keywords: Blood–Brain barrier; Matrix metalloproteinase 9; N-Methyl-D-aspartate receptor encephalitis; P38MAPK pathway; Tight junction protein.
© 2024. The Author(s).
Conflict of interest statement
Figures








References
MeSH terms
Substances
Grants and funding
- 82060236/the National Natural Science Foundation of China
- 82060236/the National Natural Science Foundation of China
- 82060236/the National Natural Science Foundation of China
- 82060236/the National Natural Science Foundation of China
- 82060236/the National Natural Science Foundation of China
- 2019GXNSFDA245032/the Natural Science Foundation of Guangxi Province
- 2019GXNSFDA245032/the Natural Science Foundation of Guangxi Province
- 2019GXNSFDA245032/the Natural Science Foundation of Guangxi Province
- 2019GXNSFDA245032/the Natural Science Foundation of Guangxi Province
- 2019GXNSFDA245032/the Natural Science Foundation of Guangxi Province
LinkOut - more resources
Full Text Sources
Miscellaneous

