GIV/Girdin Modulation of Microglial Activation in Ischemic Stroke: Impact of FTO-Mediated m6A Modification
- PMID: 39560901
- PMCID: PMC11953190
- DOI: 10.1007/s12035-024-04604-8
GIV/Girdin Modulation of Microglial Activation in Ischemic Stroke: Impact of FTO-Mediated m6A Modification
Abstract
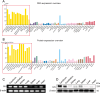
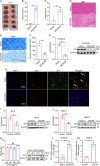
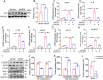
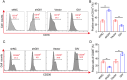
Ischemic stroke (IS) is one of the most common causes of death in the world. The lack of effective pharmacological treatments for IS was primarily due to a lack of understanding of its pathogenesis. Gα-Interacting vesicle-associated protein (GIV/Girdin) is a multi-modular signal transducer and guanine nucleotide exchange factor that controls important signaling downstream of multiple receptors. The purpose of this study was to investigate the role of GIV in IS. In the present study, we found that GIV is highly expressed in the central nervous system (CNS). GIV protein level was decreased, while GIV transcript level was increased in the middle cerebral artery occlusion reperfusion (MCAO/R) mice model. Additionally, GIV was insensitive lipopolysaccharide (LPS) exposure. Interestingly, we found that GIV overexpression dramatically restrained microglial activation, inflammatory response, and M1 polarization in BV-2 microglia induced by oxygen-glucose deprivation and reoxygenation (OGD/R). On the contrary, GIV knockdown had the opposite impact. Mechanistically, we found that GIV activated the Wnt/β-catenin signaling pathway by interacting with DVL2 (disheveled segment polarity protein 2). Notably, m6A demethylase fat mass and obesity-associated protein (FTO) decreased the N6-methyladenosine (m6A) modification-mediated increase of GIV expression and attenuated the inflammatory response in BV-2 stimulated by OGD/R. Taken together, our results demonstrate that GIV inhibited the inflammatory response via activating the Wnt/β-catenin signaling pathway which expression regulated in an FTO-mediated m6A modification in IS. These results broaden our understanding of the role of the FTO-GIV axis in IS development.
Keywords: FTO; GIV/Girdin; Inflammatory response; Ischemic stroke; M6A.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: The use and care of animals as well as all the experimental protocols were approved by the Ethic Committee of Guizhou Medical University (approval number:2000792) and were strictly in accordance with animal care and use guidelines of the National Institutes of Health. Consent to Participate: Not applicable. Consent for Publication: Consent for publication was obtained from the participants. Competing Interests: The authors declare no competing interests.
Figures

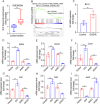


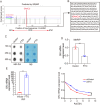
Similar articles
-
FTO alleviates cerebral ischemia/reperfusion-induced neuroinflammation by decreasing cGAS mRNA stability in an m6A-dependent manner.Cell Signal. 2023 Sep;109:110751. doi: 10.1016/j.cellsig.2023.110751. Epub 2023 Jun 14. Cell Signal. 2023. PMID: 37321527
-
USP18 Stabilized FTO Protein to Activate Mitophagy in Ischemic Stroke Through Repressing m6A Modification of SIRT6.Mol Neurobiol. 2024 Sep;61(9):6658-6674. doi: 10.1007/s12035-024-04001-1. Epub 2024 Feb 10. Mol Neurobiol. 2024. PMID: 38340205
-
Wilms' tumor 1-associated protein aggravates ischemic stroke by promoting M1 polarization of microglia by enhancing PTGS2 mRNA stability in an m6A-dependent manner.Cell Biol Int. 2025 Mar;49(3):288-302. doi: 10.1002/cbin.12266. Epub 2024 Dec 17. Cell Biol Int. 2025. PMID: 39687949 Free PMC article.
-
METTL14 Promotes Ischemic Stroke-induced Brain Injury by Stabilizing HDAC3 Expression in an m6A-IGF2BP3 Mechanism.Cell Biochem Biophys. 2025 Jun;83(2):1897-1907. doi: 10.1007/s12013-024-01596-z. Epub 2024 Oct 25. Cell Biochem Biophys. 2025. PMID: 39448421
-
Role of N6-methyladenosine modification in pathogenesis of ischemic stroke.Expert Rev Mol Diagn. 2022 Mar;22(3):295-303. doi: 10.1080/14737159.2022.2049246. Epub 2022 Mar 8. Expert Rev Mol Diagn. 2022. PMID: 35236212 Review.
References
-
- Katan M, Luft A (2018) Global Burden of Stroke. Semin Neurol 38:208–211 - PubMed
-
- Liu X, Zhang M, Liu H, Zhu R, He H, Zhou Y, Zhang Y, Li C, Liang D, Zeng Q, Huang G (2021) Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol 341:113700. 10.1016/j.expneurol.2021.113700 - PubMed
MeSH terms
Substances
Grants and funding
- 82060232/National Natural Science Foundation of China
- ZK [2021] 412/Basic Science Technology Project of Guizhou Province, China
- Qiankeheji [2020] 1Z060/Key Projects of Science and Technology Fund of Guizhou Provincial Department of Science and Technology
- Guizhou Teaching and Technology [2023]015/Department of Education of Guizhou Province(Guizhou Teaching and Technology)
LinkOut - more resources
Full Text Sources
Medical

