The PrfA regulon of Listeria monocytogenes is induced by growth in low-oxygen microaerophilic conditions
- PMID: 39560979
- PMCID: PMC11575702
- DOI: 10.1099/mic.0.001516
The PrfA regulon of Listeria monocytogenes is induced by growth in low-oxygen microaerophilic conditions
Abstract
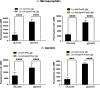
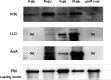
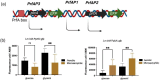
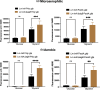
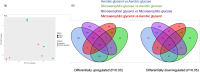
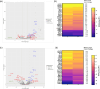
Listeria monocytogenes is a food-borne pathogen that must adapt to several environments both inside and outside the host. One such environment is the microaerophilic conditions encountered in the host intestine proximal to the mucosal surface. The aim of this study was to investigate the expression of the PrfA regulon in response to microaerophilic growth conditions in the presence of either glucose or glycerol as a carbon source using four transcriptional (Phly, PactA, P/prfA and P/plcA) gene fusions. Further, RNAseq analysis was used to identify global changes in gene expression during growth in microaerophilic conditions. Following microaerophilic growth, there was a PrfA-dependent increase in transcription from the Phly, PactA and P/plcA promoters, indicating that microaerophilic growth induces the PrfA regulon regardless of the carbon source with increased expression of the PrfA, LLO and ActA proteins. A sigB mutation had no effect on the induction of the PrfA regulon under microaerophilic conditions when glucose was used as a carbon source. In contrast, when glycerol was the carbon source, a sigB mutation increased expression from the Phly and PactA promoters regardless of the level of oxygen. The RNAseq analysis showed that 273 genes were specifically regulated by microaerophilic conditions either up or down including the PrfA regulon virulence factors. Overall, these data indicated that L. monocytogenes PrfA regulon is highly responsive to the low-oxygen conditions likely to be encountered in the small intestine and that SigB has an input into the regulation of the PrfA regulon when glycerol is the sole carbon source.
Keywords: Listeria monocytogenes; PrfA regulon; RNAseq; microaerophilic.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Species-specific differences in the activity of PrfA, the key regulator of listerial virulence genes.J Bacteriol. 2006 Nov;188(22):7941-56. doi: 10.1128/JB.00473-06. Epub 2006 Sep 15. J Bacteriol. 2006. PMID: 16980455 Free PMC article.
-
Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors.J Bacteriol. 1997 Nov;179(22):7174-80. doi: 10.1128/jb.179.22.7174-7180.1997. J Bacteriol. 1997. PMID: 9371468 Free PMC article.
-
Contributions of Listeria monocytogenes sigmaB and PrfA to expression of virulence and stress response genes during extra- and intracellular growth.Microbiology (Reading). 2006 Jun;152(Pt 6):1827-1838. doi: 10.1099/mic.0.28758-0. Microbiology (Reading). 2006. PMID: 16735745
-
Cross Talk between SigB and PrfA in Listeria monocytogenes Facilitates Transitions between Extra- and Intracellular Environments.Microbiol Mol Biol Rev. 2019 Sep 4;83(4):e00034-19. doi: 10.1128/MMBR.00034-19. Print 2019 Nov 20. Microbiol Mol Biol Rev. 2019. PMID: 31484692 Free PMC article. Review.
-
Flick of a switch: regulatory mechanisms allowing Listeria monocytogenes to transition from a saprophyte to a killer.Microbiology (Reading). 2019 Aug;165(8):819-833. doi: 10.1099/mic.0.000808. Epub 2019 May 20. Microbiology (Reading). 2019. PMID: 31107205 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

