Aiolos promotes CXCR3 expression on Th1 cells via positive regulation of IFN-γ/STAT1 signaling
- PMID: 39560988
- PMCID: PMC11721307
- DOI: 10.1172/jci.insight.180287
Aiolos promotes CXCR3 expression on Th1 cells via positive regulation of IFN-γ/STAT1 signaling
Abstract
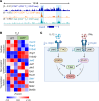
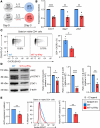
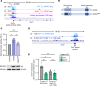
CD4+ T helper 1 (Th1) cells coordinate adaptive immune responses to intracellular pathogens, including viruses. Key to this function is the ability of Th1 cells to migrate within secondary lymphoid tissues, as well as to sites of inflammation, which relies on signals received through the chemokine receptor CXCR3. CXCR3 expression is driven by the Th1 lineage-defining transcription factor T-bet and the cytokine-responsive STAT family members STAT1 and STAT4. Here, we identify the Ikaros zinc finger (IkZF) transcription factor Aiolos (Ikzf3) as an additional positive regulator of CXCR3 both in vitro and in vivo using a murine model of influenza virus infection. Mechanistically, we found that Aiolos-deficient CD4+ T cells exhibited decreased expression of key components of the IFN-γ/STAT1 signaling pathway, including JAK2 and STAT1. Consequently, Aiolos deficiency resulted in decreased levels of STAT1 tyrosine phosphorylation and reduced STAT1 enrichment at the Cxcr3 promoter. We further found that Aiolos and STAT1 formed a positive feedback loop via reciprocal regulation of each other downstream of IFN-γ signaling. Collectively, our study demonstrates that Aiolos promotes CXCR3 expression on Th1 cells by propagating the IFN-γ/STAT1 cytokine signaling pathway.
Keywords: Cytokines; Immunology; Infectious disease; T cells; Th1 response.
Conflict of interest statement
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

