Tumor cell-derived spermidine promotes a protumorigenic immune microenvironment in glioblastoma via CD8+ T cell inhibition
- PMID: 39561012
- PMCID: PMC11735101
- DOI: 10.1172/JCI177824
Tumor cell-derived spermidine promotes a protumorigenic immune microenvironment in glioblastoma via CD8+ T cell inhibition
Abstract
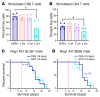
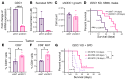
The glioblastoma (GBM) microenvironment is enriched in immunosuppressive factors that potently interfere with the function of cytotoxic T lymphocytes. Cancer cells can directly affect the immune system, but the mechanisms driving these interactions are not completely clear. Here, we demonstrate that the polyamine metabolite spermidine (SPD) was elevated in the GBM tumor microenvironment. Exogenous administration of SPD drove tumor aggressiveness in an immune-dependent manner in preclinical mouse models via reduction of CD8+ T cell frequency and reduced cytotoxic function. Knockdown of ornithine decarboxylase, the rate-limiting enzyme in SPD synthesis, did not affect cancer cell growth in vitro but did result in extended survival. Furthermore, patients with GBM with a more favorable outcome had a significant reduction in SPD compared with patients with a poor prognosis. Our results demonstrate that SPD functions as a cancer cell-derived metabolite that drives tumor progression by reducing CD8+ T cell numbers and function.
Keywords: Adaptive immunity; Brain cancer; Immunology; Oncology; Polyamines.
Conflict of interest statement
Figures





Update of
-
Tumor cell-derived spermidine promotes a pro-tumorigenic immune microenvironment in glioblastoma via CD8+ T cell inhibition.bioRxiv [Preprint]. 2023 Nov 16:2023.11.14.567048. doi: 10.1101/2023.11.14.567048. bioRxiv. 2023. Update in: J Clin Invest. 2024 Nov 19;135(2):e177824. doi: 10.1172/JCI177824. PMID: 38014234 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

