Cell non-autonomous signaling through the conserved C. elegans glycoprotein hormone receptor FSHR-1 regulates cholinergic neurotransmission
- PMID: 39561202
- PMCID: PMC11614273
- DOI: 10.1371/journal.pgen.1011461
Cell non-autonomous signaling through the conserved C. elegans glycoprotein hormone receptor FSHR-1 regulates cholinergic neurotransmission
Abstract
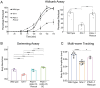
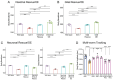
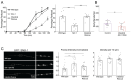
Modulation of neurotransmission is key for organismal responses to varying physiological contexts such as during infection, injury, or other stresses, as well as in learning and memory and for sensory adaptation. Roles for cell autonomous neuromodulatory mechanisms in these processes have been well described. The importance of cell non-autonomous pathways for inter-tissue signaling, such as gut-to-brain or glia-to-neuron, has emerged more recently, but the cellular mechanisms mediating such regulation remain comparatively unexplored. Glycoproteins and their G protein-coupled receptors (GPCRs) are well-established orchestrators of multi-tissue signaling events that govern diverse physiological processes through both cell-autonomous and cell non-autonomous regulation. Here, we show that follicle stimulating hormone receptor, FSHR-1, the sole Caenorhabditis elegans ortholog of mammalian glycoprotein hormone GPCRs, is important for cell non-autonomous modulation of synaptic transmission. Inhibition of fshr-1 expression reduces muscle contraction and leads to synaptic vesicle accumulation in cholinergic motor neurons. The neuromuscular and locomotor defects in fshr-1 loss-of-function mutants are associated with an underlying accumulation of synaptic vesicles, build-up of the synaptic vesicle priming factor UNC-10/RIM, and decreased synaptic vesicle release from cholinergic motor neurons. Restoration of FSHR-1 to the intestine is sufficient to restore neuromuscular activity and synaptic vesicle localization to fshr-1-deficient animals. Intestine-specific knockdown of FSHR-1 reduces neuromuscular function, indicating FSHR-1 is both necessary and sufficient in the intestine for its neuromuscular effects. Re-expression of FSHR-1 in other sites of endogenous expression, including glial cells and neurons, also restored some neuromuscular deficits, indicating potential cross-tissue regulation from these tissues as well. Genetic interaction studies provide evidence that downstream effectors gsa-1/GαS, acy-1/adenylyl cyclase and sphk-1/sphingosine kinase and glycoprotein hormone subunit orthologs, GPLA-1/GPA2 and GPLB-1/GPB5, are important for intestinal FSHR-1 modulation of the NMJ. Together, our results demonstrate that FSHR-1 modulation directs inter-tissue signaling systems, which promote synaptic vesicle release at neuromuscular synapses.
Copyright: © 2024 Buckley et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Update of
-
Cell non-autonomous signaling through the conserved C. elegans glycopeptide hormone receptor FSHR-1 regulates cholinergic neurotransmission.bioRxiv [Preprint]. 2024 Feb 12:2024.02.10.578699. doi: 10.1101/2024.02.10.578699. bioRxiv. 2024. Update in: PLoS Genet. 2024 Nov 19;20(11):e1011461. doi: 10.1371/journal.pgen.1011461. PMID: 38405708 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

