Sustained immune activation and impaired epithelial barrier integrity in the ectocervix of women with chronic HIV infection
- PMID: 39561211
- PMCID: PMC11614238
- DOI: 10.1371/journal.ppat.1012709
Sustained immune activation and impaired epithelial barrier integrity in the ectocervix of women with chronic HIV infection
Abstract
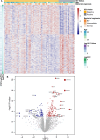
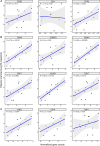
Chronic systemic immune activation significantly influences human immunodeficiency virus (HIV) disease progression. Despite evidence of a pro-inflammatory environment in the genital tract of HIV-infected women, comprehensive investigations into cervical tissue from this region remain limited. Similarly, the consequences of chronic HIV infection on the integrity of the female genital epithelium are poorly understood, despite its importance in HIV transmission and replication. Ectocervical biopsies were obtained from HIV-seropositive (n = 14) and HIV-seronegative (n = 47) female Kenyan sex workers. RNA sequencing and bioimage analysis of epithelial junction proteins (E-cadherin, desmoglein-1, claudin-1, and zonula occludens-1) were conducted, along with CD4 staining. RNA sequencing revealed upregulation of immunoregulatory genes in HIV-seropositive women, primarily associated with heightened T cell activity and interferon signaling, which further correlated with plasma viral load. Transcription factor analysis confirmed the upregulation of pro-inflammatory transcription factors, such as RELA, NFKB1, and IKZF3, which facilitates HIV persistence in T cells. Conversely, genes and pathways associated with epithelial barrier function and structure were downregulated in the context of HIV. Digital bioimage analysis corroborated these findings, revealing significant disruption of various epithelial junction proteins in ectocervical tissues of the HIV-seropositive women. Thus, chronic HIV infection associated with ectocervical inflammation, characterized by induced T cell responses and interferon signaling, coupled with epithelial disruption. These alterations may influence HIV transmission and heighten susceptibility to other sexually transmitted infections. These findings prompt exploration of therapeutic interventions to address HIV-related complications and mitigate the risk of sexually transmitted infection transmission.
Copyright: © 2024 Franzén Boger et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
P. Cz. is currently employed at DeepLife, located in 75013 Paris, France. None of the other authors declare any conflicts of interest.
Figures



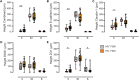
Similar articles
-
Spatial transcriptomics and in situ immune cell profiling of the host ectocervical landscape of HIV infected Kenyan sex working women.Front Immunol. 2024 Dec 2;15:1483346. doi: 10.3389/fimmu.2024.1483346. eCollection 2024. Front Immunol. 2024. PMID: 39687623 Free PMC article.
-
Stable CD4 expression and local immune activation in the ectocervical mucosa of HIV-infected women.J Immunol. 2013 Oct 1;191(7):3948-54. doi: 10.4049/jimmunol.1301220. Epub 2013 Sep 4. J Immunol. 2013. PMID: 24006463
-
Multi-omics analysis of the cervical epithelial integrity of women using depot medroxyprogesterone acetate.PLoS Pathog. 2022 May 9;18(5):e1010494. doi: 10.1371/journal.ppat.1010494. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35533147 Free PMC article.
-
HIV-1 infection in the female reproductive tract: role of interactions between HIV-1 and genital epithelial cells.Am J Reprod Immunol. 2011 Mar;65(3):253-60. doi: 10.1111/j.1600-0897.2010.00965.x. Epub 2011 Jan 12. Am J Reprod Immunol. 2011. PMID: 21223427 Review.
-
Potential mechanisms for increased HIV-1 transmission across the endocervical epithelium during C. trachomatis infection.Curr HIV Res. 2012 Apr;10(3):218-27. doi: 10.2174/157016212800618093. Curr HIV Res. 2012. PMID: 22384841 Free PMC article. Review.
Cited by
-
HIV-associated penile anaerobes disrupt epithelial barrier integrity.PLoS Pathog. 2025 Apr 17;21(4):e1013094. doi: 10.1371/journal.ppat.1013094. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40245064 Free PMC article.
References
-
- So-Armah KA, Tate JP, Chang CCH, Butt AA, Gerschenson M, Gibert CL, et al.. Do Biomarkers of Inflammation, Monocyte Activation, and Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? J Acquir Immune Defic Syndr. 2016. Jun 6;72(2):206. Available from: /pmc/articles/PMC4867134/. doi: 10.1097/QAI.0000000000000954 - DOI - PMC - PubMed
-
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al.. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Medicine 2006 12:12. 2006. Nov 19;12(12):1365–71. Available from: https://www.nature.com/articles/nm1511. doi: 10.1038/nm1511 - DOI - PubMed
-
- Giorgi J V., Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al.. Shorter Survival in Advanced Human Immunodeficiency Virus Type 1 Infection Is More Closely Associated with T Lymphocyte Activation than with Plasma Virus Burden or Virus Chemokine Coreceptor Usage. J Infect Dis. 1999. Apr 1;179(4):859–70. Available from: doi: 10.1086/314660 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

