Lewis-Acid Mediated Reactivity in Single-Molecule Junctions
- PMID: 39561214
- PMCID: PMC11622238
- DOI: 10.1021/jacs.4c14176
Lewis-Acid Mediated Reactivity in Single-Molecule Junctions
Abstract
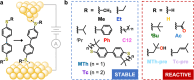
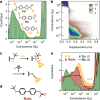
While chemical reactions at a gold electrode can be monitored using molecular conductance and driven by extrinsic stimuli, the intrinsic properties of the nanostructured interface may perform important additional functions that are not yet well understood. Here we evaluate these properties in studies of single-molecule junctions formed from components comprising 4,4'-biphenyl backbones functionalized with 12 different sulfur-based linker groups. With some linkers, we find evidence for in situ S-C(sp3) bond breaking, and C(sp2)-C(sp3) bond forming, reactions consistent with the ex situ transformations expected for those groups in the presence of a Lewis acid. Notably, we also approach the limits of substituent influence on the conductance of physisorbed sulfur-linked junctions. As an illustrative example, we show that a tert-butylthio-functionalized precursor can form both chemisorbed (Au-S) junctions, consistent with heterolytic S-C(sp3) bond cleavage and generation of a stable tert-butyl carbocation, as well as physisorbed junctions that are >1 order of magnitude lower conductance than analogous junctions comprising cyclic "locked" thioether contacts. These findings are supported by a systematic analysis of model thioether components comprising different simple hydrocarbon substituents of intermediate size, which do not form chemisorbed contacts and further clarify the inverse relationship between conductance and substituent steric bulk. First-principles calculations confirm that bulky sulfur-substituents increase the probability of forming junction geometries with reduced electronic coupling between the electrode and π-conjugated molecular backbone. Together, this work helps to rationalize the dual roles that linker chemical structure and metal electrode Lewis character can play in mediating interfacial reactions in break-junction experiments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

