Predicting Pain Response to a Remote Musculoskeletal Care Program for Low Back Pain Management: Development of a Prediction Tool
- PMID: 39561359
- PMCID: PMC11615557
- DOI: 10.2196/64806
Predicting Pain Response to a Remote Musculoskeletal Care Program for Low Back Pain Management: Development of a Prediction Tool
Abstract
Background: Low back pain (LBP) presents with diverse manifestations, necessitating personalized treatment approaches that recognize various phenotypes within the same diagnosis, which could be achieved through precision medicine. Although prediction strategies have been explored, including those employing artificial intelligence (AI), they still lack scalability and real-time capabilities. Digital care programs (DCPs) facilitate seamless data collection through the Internet of Things and cloud storage, creating an ideal environment for developing and implementing an AI predictive tool to assist clinicians in dynamically optimizing treatment.
Objective: This study aims to develop an AI tool that continuously assists physical therapists in predicting an individual's potential for achieving clinically significant pain relief by the end of the program. A secondary aim was to identify predictors of pain nonresponse to guide treatment adjustments.
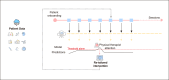
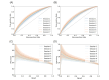
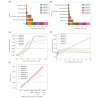
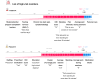
Methods: Data collected actively (eg, demographic and clinical information) and passively in real-time (eg, range of motion, exercise performance, and socioeconomic data from public data sources) from 6125 patients enrolled in a remote digital musculoskeletal intervention program were stored in the cloud. Two machine learning techniques, recurrent neural networks (RNNs) and light gradient boosting machine (LightGBM), continuously analyzed session updates up to session 7 to predict the likelihood of achieving significant pain relief at the program end. Model performance was assessed using the area under the receiver operating characteristic curve (ROC-AUC), precision-recall curves, specificity, and sensitivity. Model explainability was assessed using SHapley Additive exPlanations values.
Results: At each session, the model provided a prediction about the potential of being a pain responder, with performance improving over time (P<.001). By session 7, the RNN achieved an ROC-AUC of 0.70 (95% CI 0.65-0.71), and the LightGBM achieved an ROC-AUC of 0.71 (95% CI 0.67-0.72). Both models demonstrated high specificity in scenarios prioritizing high precision. The key predictive features were pain-associated domains, exercise performance, motivation, and compliance, informing continuous treatment adjustments to maximize response rates.
Conclusions: This study underscores the potential of an AI predictive tool within a DCP to enhance the management of LBP, supporting physical therapists in redirecting care pathways early and throughout the treatment course. This approach is particularly important for addressing the heterogeneous phenotypes observed in LBP.
Trial registration: ClinicalTrials.gov NCT04092946; https://clinicaltrials.gov/ct2/show/NCT04092946 and NCT05417685; https://clinicaltrials.gov/ct2/show/NCT05417685.
Keywords: artificial intelligence; clinical decision support; machine learning; personalized medicine; predictive modeling; rehabilitation; telerehabilitation.
©Anabela C Areias, Robert G Moulder, Maria Molinos, Dora Janela, Virgílio Bento, Carolina Moreira, Vijay Yanamadala, Steven P Cohen, Fernando Dias Correia, Fabíola Costa. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 19.11.2024.
Conflict of interest statement
Conflicts of Interest: The authors declare the following competing financial interests: ACA, RGM, MM, DJ, CM, VY, FDC, and FC are employees of Sword Health Inc, the sponsor of this study. FDC, VY, and VB also hold equity in Sword Health Inc. SPC is an independent scientific and clinical consultant who received advisory honoraria from Sword Health.
Figures

References
-
- Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021 Dec 19;396(10267):2006–2017. doi: 10.1016/S0140-6736(20)32340-0. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(20)32340-0 S0140-6736(20)32340-0 - DOI - PMC - PubMed
-
- Cholewicki J, Breen A, Popovich JM, Reeves NP, Sahrmann SA, van Dillen LR, Vleeming A, Hodges PW. Can biomechanics research lead to more effective treatment of low back pain? A point-counterpoint debate. J Orthop Sports Phys Ther. 2019 Jun;49(6):425–436. doi: 10.2519/jospt.2019.8825. https://europepmc.org/abstract/MED/31092123 - DOI - PMC - PubMed
-
- Lin I, Wiles L, Waller R, Goucke R, Nagree Y, Gibberd M, Straker L, Maher CG, O'Sullivan PPB. What does best practice care for musculoskeletal pain look like? Eleven consistent recommendations from high-quality clinical practice guidelines: systematic review. Br J Sports Med. 2020 Jan;54(2):79–86. doi: 10.1136/bjsports-2018-099878.bjsports-2018-099878 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

