Consensus recommendations from the 2024 Lymphoma Research Foundation workshop on treatment selection and sequencing in CLL or SLL
- PMID: 39561376
- PMCID: PMC11993837
- DOI: 10.1182/bloodadvances.2024014474
Consensus recommendations from the 2024 Lymphoma Research Foundation workshop on treatment selection and sequencing in CLL or SLL
Abstract
Over the past decade, treatment recommendations for patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) have shifted from traditional chemoimmunotherapy to targeted therapies. Multiple new therapies are commercially available, and, in many cases, a lack of randomized clinical trial data makes selection of the optimal treatment for each patient challenging. Additionally, many patients continue to receive chemoimmunotherapy in the United States, suggesting a gap between guidelines and real-world practice. The Lymphoma Research Foundation convened a workshop comprising a panel of CLL/SLL experts in the United States to develop consensus recommendations for selection and sequencing of therapies for patients with CLL/SLL in the United States. Herein, the recommendations are compiled for use as a practical clinical guide for treating providers caring for patients with CLL/SLL, which complement existing guidelines by providing a nuanced discussion relating how our panel of CLL/SLL experts in the United States care for patients in a real-world environment.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.D.S. received consulting fees from AstraZeneca, Bristol Myers Squibb, Genentech/Roche, and Loxo@Lilly; and received research funding from Adaptive Biotechnologies, BeiGene, BostonGene, Genentech/Roche, GlaxoSmithKline, Moderna, Takeda, and TG Therapeutics. I.A. received consulting fees from AstraZeneca, BeiGene, and Loxo@Lilly. J.R.B. received consulting fees from AbbVie, Acerta/AstraZeneca, Alloplex Biotherapeutics, BeiGene, Galapagos, Genentech/Roche, Grifols Worldwide Operations, Hutchmed, InnoCare Pharma Inc, iOnctura, Janssen, Kite, Loxo/Lilly, MEI Pharma, Merck, Numab Therapeutics, Pfizer, and Pharmacyclics; and received research funding from BeiGene, Gilead, iOnctura, Loxo/Lilly, MEI Pharma, SecuraBio, and TG Therapeutics. J.B. received consulting fees from AbbVie, AstraZeneca, BeiGene, Janssen; and received research funding from AbbVie, Pharmacyclics, and Schrodinger. C.C. received consulting fees from AbbVie, AstraZeneca, BeiGene, Octapharma, Lilly, MEI Pharma, TG Therapeutics, Janssen, Genentech, Allogene, Mingsight; received research funding from AbbVie, CarnaBio, and Lilly; received payments for lectures from AbbVie, Genentech, AstraZeneca, and BeiGene; received payment for developing educational materials from Achilles Therapeutics Aptitute Health, BioAscend, Cardinal Health, Clinical Care Options, Curio, DAVA Oncology Mashup, MJH Life Sciences National Association of Continuing Education OncLive, Oncoboard, Physicians Education Resource Peerview, PRIME Education, LLC Prova Education, and Targeted Oncology; holds stock in Pfizer and Bluebird Bio; and received fees for serving on data monitoring boards from Octapharma and AbbVie. M.H. received consulting fees from AbbVie, ADC Therapeutics, BeiGene, Pharmacyclics, Genentech, AstraZeneca, Kite, and Novartis; received fees for serving on data monitoring boards from Novartis; and received research support from Genentech. R.J. received consulting fees from AbbVie, Galapagos, Pharmacyclics, Janssen, SecuraBio, BeiGene, Genentech, AstraZeneca, and Lilly; received travel expenses from AstraZeneca; and received research support from AbbVie, Pharmacyclics, AstraZeneca, and Lilly. A.K. received consulting fees from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Janssen, Kite, Eli Lilly, and Pharmacyclics; and received payment for lectures from AbbVie, AstraZeneca, and BeiGene, and payment for development of educational materials from Bristol Myers Squibb and Janssen. A.L. received consulting fees from AbbVie, Eli Lilly, AstraZeneca, and BeiGene. K.P. received consulting fees from AstraZeneca, AbbVie, ADC therapeutics, BeiGene, Bristol Myers Squibb, Caribou, Fate Therapeutics, Genentech, Janssen, Kite, Lilly, Merck, Nurix, Pfizer, Pharmacyclics, Sana, and Xencor; received research funding from AstraZeneca; received travel expenses from Lilly; and received payment for lectures from AstraZeneca and Kite. J.R. received consulting fees from AbbVie, ADC Therapeutics, BeiGene, Epizyme, Genentech, Janssen, MorphoSys, Pharmacyclics, SeaGen, TG Therapeutics, and Verastem; and received research funding from AbbVie, Acerta, Janssen, Loxo Oncology, Oncternal Therapeutics, Pharmacyclics, and VelosBio. H.S. received consulting fees from Seattle Genetics, ADC Therapeutics and AstraZeneca. A.S. received consulting fees from Alexion, AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Janssen, Kite Pharma, Lilly, SeaGen, and Pharmacyclics; and received payment for lectures from AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Genentech, Kite Pharma, Janssen, Lilly, GenMab, Jazz Pharmaceuticals, Pharmacyclics, and SeaGen. M.T. received consulting fees from AstraZeneca, BeiGene, Janssen/Pharmacyclics, AbbVie, Loxo Oncology; received research support from BeiGene, AstraZeneca, Genmab, Nurix Therapeutics, Genentech, and AbbVie; received payment for development of educational materials and/or speaking from Peerview Learning Institute, Phillips Group Oncology Communications, DAVA Oncology, Loxo Oncology, eScientiq and Brazillian Association of Hematology, Hemotherapy, and Cellular Therapy; received travel expenses from Nurix Therapeutics, Genmab, and DAVA Oncology; and received honoraria from VJHemOnc, MGH Life Sciences, Intellisphere LLC, and Curio Science. D.M.S. received consulting fees from AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Pharmacyclics, Eli Lilly, Bristol Myers Squibb, and Celgene; and received research support from Novartis and AstraZeneca. The remaining authors declare no competing financial interests.
D.E., P.R., and H.S. are scholars of the current Scholars of the Lymphoma Scientific Research Mentoring Program, Lymphoma Research Foundation.
Figures
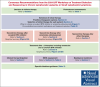


Comment in
-
Consensus in CLL: global needs matter.Blood Adv. 2025 Mar 11;9(5):1210-1212. doi: 10.1182/bloodadvances.2024015355. Blood Adv. 2025. PMID: 40067336 Free PMC article. No abstract available.
References
-
- Lucero KT, Frei CR, Ryan KJ, et al. Shift in first-line therapies for United States Veterans Health Administration (VHA) patients with chronic lymphocytic leukemia (CLL) from 2014 to 2021. Blood. 2022;140(suppl 1):9915–9916.
-
- Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. - PubMed
-
- Dighiero G, Maloum K, Desablens B, et al. Chlorambucil in indolent chronic lymphocytic leukemia. French Cooperative Group on chronic lymphocytic leukemia. N Engl J Med. 1998;338(21):1506–1514. - PubMed
-
- Hoechstetter MA, Busch R, Eichhorst B, et al. Early, risk-adapted treatment with fludarabine in Binet stage A chronic lymphocytic leukemia patients: results of the CLL1 trial of the German CLL study group. Leukemia. 2017;31(12):2833–2837. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

