Current Status of Co-Ordering of C-Reactive Protein and Erythrocyte Sedimentation Rate Testing in Korea
- PMID: 39561808
- PMCID: PMC11576217
- DOI: 10.3346/jkms.2024.39.e319
Current Status of Co-Ordering of C-Reactive Protein and Erythrocyte Sedimentation Rate Testing in Korea
Abstract
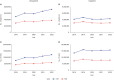
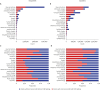
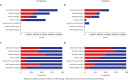
We retrospectively examined current trends in ordering for erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) testing. All claims corresponding to ESR and CRP testing for hospital visits in 2022 were obtained from a platform operated by the Health Insurance and Review Agency. The annual (2018-2022) utilization and cost of ESR and CRP, total inpatient days, and patient encounters with outpatients were retrieved. The number of ESR and CRP tests gradually increased over 5 years, except a slight decrease in 2020. The proportion of claims with co-ordering of ESR and CRP tests was 46.64%. More than 60% co-ordering claims were observed in orthopedic surgery, neurosurgery, and plastic surgery departments. The proportion of co-orders was relatively high in inpatient setting and primary hospitals. This study indicated frequent co-ordering patterns of ESR and CRP tests, highlighting an urgent need for diagnostic stewardship programs on ESR and CRP testing in Korea.
Keywords: C-Reactive Protein; Choosing Wisely; Co-ordering; Erythrocyte Sedimentation Rate; Laboratory Stewardship.
© 2024 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
-
- Colombet I, Pouchot J, Kronz V, Hanras X, Capron L, Durieux P, et al. Agreement between erythrocyte sedimentation rate and C-reactive protein in hospital practice. Am J Med. 2010;123(9):863.e7–863.e13. - PubMed
-
- Feldman M, Aziz B, Kang GN, Opondo MA, Belz RK, Sellers C. C-reactive protein and erythrocyte sedimentation rate discordance: frequency and causes in adults. Transl Res. 2013;161(1):37–43. - PubMed
-
- American Society for Clinical Pathology. Thirty five things physicians and patients should question. [Updated 2020]. [Accessed May 1, 2024]. https://www.choosingwisely.org/societies/american-society-for-clinical-p...
-
- Korean Society for Laboratory Medicine. Laboratory test utilization management: appropriateness of ESR and CRP test order. [Updated 2020]. [Accessed May 1, 2024]. https://www.kslm.org/eNewsletter/2020_03/newsletter_51.pdf?v=2004290609 .
-
- Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–728. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

