TNFR2 blockade promotes antitumoral immune response in PDAC by targeting activated Treg and reducing T cell exhaustion
- PMID: 39562007
- PMCID: PMC11580249
- DOI: 10.1136/jitc-2024-008898
TNFR2 blockade promotes antitumoral immune response in PDAC by targeting activated Treg and reducing T cell exhaustion
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive cancers, highly resistant to standard chemotherapy and immunotherapy. Regulatory T cells (Tregs) expressing tumor necrosis factor α receptor 2 (TNFR2) contribute to immunosuppression in PDAC. Treg infiltration correlates with poor survival and tumor progression in patients with PDAC. We hypothesized that TNFR2 inhibition using a blocking monoclonal antibody (mAb) could shift the Treg-effector T cell balance in PDAC, thus enhancing antitumoral responses.
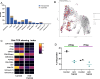
Method: To support this hypothesis, we first described TNFR2 expression in a cohort of 24 patients with PDAC from publicly available single-cell analysis data. In orthotopic and immunocompetent mouse models of PDAC, we also described the immune environment of PDAC after immune cell sorting and single-cell analysis. The modifications of the immune environment before and after anti-TNFR2 mAb treatment were evaluated as well as the effect on tumor progression.
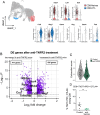
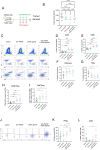
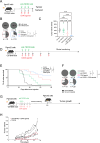
Results: Patients with PDAC exhibited elevated TNFR2 expression in Treg, myeloid cells and endothelial cells and lower level in tumor cells. By flow cytometry and single-cell RNA-seq analysis, we identified two Treg populations in orthotopic mouse models: Resting and activated Tregs. The anti-TNFR2 mAb selectively targeted activated tumor-infiltrating Tregs, reducing T cell exhaustion markers in CD8+ T cells. However, anti-TNFR2 treatment alone had limited efficacy in activating CD8+ T cells and only slightly reduced the tumor growth. The combination of the anti-TNFR2 mAb with agonistic anti-CD40 mAb promoted stronger T cell activation, tumor growth inhibition, and improved survival and immunological memory in PDAC-bearing mice.
Conclusion: Our data suggest that combining a CD40 agonist with a TNFR2 antagonist represents a promising therapeutic strategy for patients with PDAC.
Keywords: Adenocarcinoma; Immune modulatory; T regulatory cell - Treg; Tumor infiltrating lymphocyte - TIL; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: EP is co-founder and consultant for Egle-Tx.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
