High-Density Recording Reveals Sparse Clusters (But Not Columns) for Shape and Texture Encoding in Macaque V4
- PMID: 39562041
- PMCID: PMC11780345
- DOI: 10.1523/JNEUROSCI.1893-23.2024
High-Density Recording Reveals Sparse Clusters (But Not Columns) for Shape and Texture Encoding in Macaque V4
Abstract
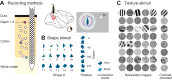
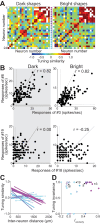
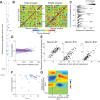
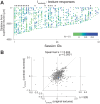
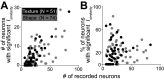
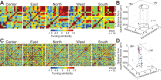
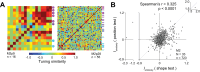
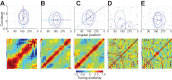
Macaque area V4 includes neurons that exhibit exquisite selectivity for visual form and surface texture, but their functional organization across laminae is unknown. We used high-density Neuropixels probes in two awake monkeys (one female and one male) to characterize the shape and texture tuning of dozens of neurons simultaneously across layers. We found sporadic clusters of neurons that exhibit similar tuning for shape and texture: ∼20% exhibited similar tuning with their neighbors. Importantly, these clusters were confined to a few layers, seldom "columnar" in structure. This was the case even when neurons were strongly driven and exhibited robust contrast invariance for shape and texture tuning. We conclude that functional organization in area V4 is not columnar for shape and texture stimulus features and in general organization may be at a coarser stimulus category scale (e.g., selectivity for stimuli with vs without 3D cues) and a coarser spatial scale (assessed by optical imaging), rather than at a fine scale in terms of similarity in single-neuron tuning for specific features. We speculate that this may be a direct consequence of the great diversity of inputs integrated by V4 neurons to build variegated tuning manifolds in a high-dimensional space.
Keywords: functional architecture; monkey; neuropixels; object recognition; shape perception; visual cortex.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Update of
-
High-density recording reveals sparse clusters (but not columns) for shape and texture encoding in macaque V4.bioRxiv [Preprint]. 2023 Oct 17:2023.10.15.562424. doi: 10.1101/2023.10.15.562424. bioRxiv. 2023. Update in: J Neurosci. 2025 Jan 29;45(5):e1893232024. doi: 10.1523/JNEUROSCI.1893-23.2024. PMID: 37904996 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
