Cost-utility of cochlear implantation in single-sided deafness and asymmetric hearing loss: results of a randomized controlled trial
- PMID: 39562492
- PMCID: PMC12204948
- DOI: 10.1007/s10198-024-01740-9
Cost-utility of cochlear implantation in single-sided deafness and asymmetric hearing loss: results of a randomized controlled trial
Abstract
Objectives: To determine the Incremental Cost-Utility Ratio (ICUR) of cochlear implantation in the treatment of adult patients with single-sided deafness (SSD) and asymmetric hearing loss (AHL).
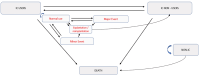
Methods: This prospective multicenter pragmatic study including a randomized controlled trial (RCT) enrolled 155 subjects with SSD or AHL. Subjects chose a treatment option between: abstention, Contralateral Routing Of the Signal hearing aids, Bone Conduction Device or Cochlear Implant (CI). Participants who opted for CI were then randomized between two arms: "immediate CI" where the cochlear implantation was performed within one month and "initial observation" where subjects were first observed. The ICUR of CI was determined at 6 months follow-up by comparing the two arms. Utility was measured using EuroQoL- 5 dimensions (EQ-5D), to calculate the gain in Quality-Adjusted Life Years (QALY). Individual costs were extracted from the French National Health Insurance database. A Markovian MultiState (MMS) model assessed the ICUR evolution over the lifetime horizon.
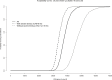
Results: Among the 155 included participants, 51 opted for a CI and were randomized. For a 6 months follow-up period, the ICUR was €422,279/QALY gained after CI. Using the MMS model, the ICUR of CI decreased to €57,561/QALY at 10 years follow-up, €38,006/QALY at 20 years, and dropped to €26,715 at 50 years. In the participants with severe tinnitus, mean ICUR was €31,105/QALY at 10 years.
Conclusions: CI can be considered as an efficient treatment in SSD and AHL from 20 years follow-up in the global population, and before 10 years follow-up in patients with severe associated tinnitus.
Keywords: Asymmetric hearing loss; Cochlear implant; ICUR; Single-sided deafness; Tinnitus.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no conflicts of interest to declare.
Figures

References
-
- Borre, E.D., Kaalund, K., Frisco, N., Zhang, G., Ayer, A., Kelly-Hedrick, M., Reed, S.D., Emmett, S.D., Francis, H., Tucci, D.L., Wilson, B.S., Kosinski, A.S., Ogbuoji, O., Sanders Schmidler, G.D.: The impact of hearing loss and its treatment on Health-Related Quality of Life Utility: A systematic review with Meta-analysis. J. Gen. Intern. Med. 38(2), 456–479 (2023). 10.1007/s11606-022-07795-9 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

